386 results found for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16 | Next | View 100 per page
Low relevance matches: 424 other results may be of interest to you. Show low relevance matches
Forces and Moving - The way objects move depends on a variety of factors including their size and shape ACSSU031 Year 2 Chemical Sciences
Materials - Different materials can be combined, including by mixing, for a particular purpose ACSSU033 Year 2 Physical Sciences
Forces and Moving - A push or a pull affects how an object moves or changes shape ACSSU076 Year 4 Physical Sciences
Forces and Moving - Forces can be exerted by one object on another through direct contact or from a distance ACSSU080 Year 5 Physical Sciences
Light and Sound - Light from a source forms shadows and can be absorbed, reflected and refracted ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU117 Year 7 Physical Sciences
Forces and Machines - Change to an object’s motion is caused by unbalanced forces, including Earth’s gravitational attraction, acting on the object ACSSU153 Year 8 Earth and Space Sciences
Rocks and Minerals - Sedimentary, igneous and metamorphic rocks contain minerals and are formed by processes that occur within Earth over a variety of timescales ACSSU155 Year 8 Physical Sciences
Energy Forms - Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems ACSSU178 Year 9 Chemical Sciences
Chemical Reactions - Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created or destroyed ACSSU225 Year 8 Chemical Sciences
Chemical Reactions - Chemical change involves substances reacting to form new substances ACSSU190 Year 10 Physical Sciences
Energy Conservation - Energy conservation in a system can be explained by describing energy transfers and transformations ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL115 Year 12 Maintaining the internal environment
Homeostasis - Animals, whether osmo-regulators or osmo-conformers, and plants, have various mechanisms to maintain water balance that involve structural features, and behavioural, physiological and homeostatic responses ACSCH018 Year 11 Chemical fundamentals
Properties and structure of atoms - Atoms can be modelled as a nucleus surrounded by electrons in distinct energy levels, held together by electrostatic forces of attraction between the nucleus and electrons; atoms can be represented using electron shell diagrams (all electron shells or val ACSCH025 Year 11 Chemical fundamentals
Properties and structure of materials - Materials are either pure substances with distinct measurable properties (for example, melting and boiling point, reactivity, strength, density) or mixtures with properties dependent on the identity and relative amounts of the substances that make up the ACSCH030 Year 11 Chemical fundamentals
Properties and structure of materials - Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons and protons; ions are represented by formulae which include the number of constituent atoms and the charge of the ion (for example, O2–, SO42–) ACSCH032 Year 11 Chemical fundamentals
Properties and structure of materials - The characteristic properties of metals (for example, malleability, thermal conductivity, electrical conductivity) are explained by modelling metallic bonding as a regular arrangement of positive ions (cations) made stable by electrostatic forces of attra ACSCH036 Year 11 Chemical fundamentals
Chemical reactions - All chemical reactions involve the creation of new substances and associated energy transformations, commonly observable as changes in the temperature of the surroundings and/or the emission of light ACSCH037 Year 11 Chemical fundamentals
Chemical reactions - Endothermic and exothermic reactions can be explained in terms of the Law of Conservation of Energy and the breaking and reforming of bonds; heat energy released or absorbed can be represented in thermochemical equations ACSCH039 Year 11 Chemical fundamentals
Chemical reactions - A mole is a precisely defined quantity of matter equal to Avogadro’s number of particles; the mole concept and the Law of Conservation of Mass can be used to calculate the mass of reactants and products in a chemical reaction ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH059 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - Data from chromatography techniques (for example, thin layer, gas and highperformance liquid chromatography) can be used to determine the composition and purity of substances; the separation of the components is caused by the variation of strength of the ACSCH060 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The behaviour of gases, including the qualitative relationships between pressure, temperature and volume, can be explained using kinetic theory ACSCH065 Year 11 Molecular interactions and reactions
Aqueous solutions and acidity - The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature ACSCH069 Year 11 Molecular interactions and reactions
Rates of chemical reactions - The rate of chemical reactions can be quantified by measuring the rate of formation of products or the depletion of reactants ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH131 Year 12 Structure synthesis and design
Chemical synthesis and design - Chemical synthesis involves the selection of particular reagents to form a product with specific properties (for example, pharmaceuticals, fuels, cosmetics, cleaning products) ACSPH039 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is conserved in the energy transfers and transformations that occur in an electrical circuit ACSPH042 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Power is the rate at which energy is transformed by a circuit component; power enables quantitative analysis of energy transformations in the circuit ACSPH043 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Resistance for ohmic and nonohmic components is defined as the ratio of potential difference across the component to the current in the component ACSPH060 Year 11 Linear Motion and Waves
Linear motion and force - Uniformly accelerated motion is described in terms of relationships between measurable scalar and vector quantities, including displacement, speed, velocity and acceleration ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH062 Year 11 Linear Motion and Waves
Linear motion and force - Vertical motion is analysed by assuming the acceleration due to gravity is constant near Earth’s surface ACSPH063 Year 11 Linear Motion and Waves
Linear motion and force - Newton’s Three Laws of Motion describe the relationship between the force or forces acting on an object, modelled as a point mass, and the motion of the object due to the application of the force or forces ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH066 Year 11 Linear Motion and Waves
Linear motion and force - Collisions may be elastic and inelastic; kinetic energy is conserved in elastic collisions ACSPH072 Year 11 Linear Motion and Waves
Waves - The superposition of waves in a medium may lead to the formation of standing waves and interference phenomena, including standing waves in pipes and on stretched strings ACSPH102 Year 12 Gravity and electromagnetism
Electromagnetism - Electrostatically charged objects exert a force upon one another; the magnitude of this force can be calculated using Coulomb’s Law ACSPH110 Year 12 Gravity and electromagnetism
Electromagnetism - A changing magnetic flux induces a potential difference; this process of electromagnetic induction is used in stepup and stepdown transformers, DC and AC generators, and AC induction motors ACSBL053 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Cellular respiration is a biochemical process that occurs in different locations in the cytosol and mitochondria and metabolises organic compounds, aerobically or anaerobically, to release useable energy in the form of ATP; the overall process can be repr ACSPH108 Year 12 Gravity and electromagnetism
Electromagnetism - Magnets, magnetic materials, moving charges and currentcarrying wires experience a force in a magnetic field; this force is utilised in DC electric motors ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system ACSPH100 Year 12 Gravity and electromagnetism
Gravity and motion - When an object experiences a net force of constant magnitude perpendicular to its velocity, it will undergo uniform circular motion, including circular motion on a horizontal plane and around a banked track ACSCH031 Year 11 Chemical fundamentals
Properties and structure of materials - The properties of ionic compounds (for example, high melting point, brittleness, ability to conduct electricity when liquid or in solution) are explained by modelling ionic bonding as ions arranged in a crystalline lattice structure with forces of attract ACSCH137 Year 12 Structure synthesis and design
Chemical synthesis and design - Fuels (for example, biodiesel, ethanol, hydrogen) can be synthesised from organic or inorganic sources using a range of chemical reactions including addition, oxidation and esterification ACSPH075 Year 11 Linear Motion and Waves
Waves - A ray model of light may be used to describe reflection, refraction and image formation from lenses and mirrors ACSPH077 Year 11 Linear Motion and Waves
Waves - The speed of light is finite and many orders of magnitude greater than the speed of mechanical waves (for example, sound and water waves); its intensity decreases in an inverse square relationship with distance from a point source ACSPH098 Year 12 Gravity and electromagnetism
Gravity and motion - The vector nature of the gravitational force can be used to analyse motion on inclined planes by considering the components of the gravitational force (that is, weight) parallel and perpendicular to the plane ACSPH103 Year 12 Gravity and electromagnetism
Electromagnetism - A positively charged body placed in an electric field will experience a force in the direction of the field; the strength of the electric field is defined as the force per unit charge ACSPH104 Year 12 Gravity and electromagnetism
Electromagnetism - Point charges and charged objects produce an electric field in the space that surrounds them; field theory attributes the electrostatic force on a point charge or charged body to the presence of an electric field ACSPH105 Year 12 Gravity and electromagnetism
Electromagnetism - When a charged body moves or is moved from one point to another in an electric field and its potential energy changes, work is done on or by the field
















386 results found for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16 | Next | View 100 per page
Low relevance matches: 424 other results may be of interest to you. Show low relevance matches
Curriculum resources related to 'for'
ACSSU005 Foundation Physical SciencesForces and Moving - The way objects move depends on a variety of factors including their size and shape ACSSU031 Year 2 Chemical Sciences
Materials - Different materials can be combined, including by mixing, for a particular purpose ACSSU033 Year 2 Physical Sciences
Forces and Moving - A push or a pull affects how an object moves or changes shape ACSSU076 Year 4 Physical Sciences
Forces and Moving - Forces can be exerted by one object on another through direct contact or from a distance ACSSU080 Year 5 Physical Sciences
Light and Sound - Light from a source forms shadows and can be absorbed, reflected and refracted ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU117 Year 7 Physical Sciences
Forces and Machines - Change to an object’s motion is caused by unbalanced forces, including Earth’s gravitational attraction, acting on the object ACSSU153 Year 8 Earth and Space Sciences
Rocks and Minerals - Sedimentary, igneous and metamorphic rocks contain minerals and are formed by processes that occur within Earth over a variety of timescales ACSSU155 Year 8 Physical Sciences
Energy Forms - Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems ACSSU178 Year 9 Chemical Sciences
Chemical Reactions - Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created or destroyed ACSSU225 Year 8 Chemical Sciences
Chemical Reactions - Chemical change involves substances reacting to form new substances ACSSU190 Year 10 Physical Sciences
Energy Conservation - Energy conservation in a system can be explained by describing energy transfers and transformations ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL115 Year 12 Maintaining the internal environment
Homeostasis - Animals, whether osmo-regulators or osmo-conformers, and plants, have various mechanisms to maintain water balance that involve structural features, and behavioural, physiological and homeostatic responses ACSCH018 Year 11 Chemical fundamentals
Properties and structure of atoms - Atoms can be modelled as a nucleus surrounded by electrons in distinct energy levels, held together by electrostatic forces of attraction between the nucleus and electrons; atoms can be represented using electron shell diagrams (all electron shells or val ACSCH025 Year 11 Chemical fundamentals
Properties and structure of materials - Materials are either pure substances with distinct measurable properties (for example, melting and boiling point, reactivity, strength, density) or mixtures with properties dependent on the identity and relative amounts of the substances that make up the ACSCH030 Year 11 Chemical fundamentals
Properties and structure of materials - Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons and protons; ions are represented by formulae which include the number of constituent atoms and the charge of the ion (for example, O2–, SO42–) ACSCH032 Year 11 Chemical fundamentals
Properties and structure of materials - The characteristic properties of metals (for example, malleability, thermal conductivity, electrical conductivity) are explained by modelling metallic bonding as a regular arrangement of positive ions (cations) made stable by electrostatic forces of attra ACSCH036 Year 11 Chemical fundamentals
Chemical reactions - All chemical reactions involve the creation of new substances and associated energy transformations, commonly observable as changes in the temperature of the surroundings and/or the emission of light ACSCH037 Year 11 Chemical fundamentals
Chemical reactions - Endothermic and exothermic reactions can be explained in terms of the Law of Conservation of Energy and the breaking and reforming of bonds; heat energy released or absorbed can be represented in thermochemical equations ACSCH039 Year 11 Chemical fundamentals
Chemical reactions - A mole is a precisely defined quantity of matter equal to Avogadro’s number of particles; the mole concept and the Law of Conservation of Mass can be used to calculate the mass of reactants and products in a chemical reaction ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH059 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - Data from chromatography techniques (for example, thin layer, gas and highperformance liquid chromatography) can be used to determine the composition and purity of substances; the separation of the components is caused by the variation of strength of the ACSCH060 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The behaviour of gases, including the qualitative relationships between pressure, temperature and volume, can be explained using kinetic theory ACSCH065 Year 11 Molecular interactions and reactions
Aqueous solutions and acidity - The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature ACSCH069 Year 11 Molecular interactions and reactions
Rates of chemical reactions - The rate of chemical reactions can be quantified by measuring the rate of formation of products or the depletion of reactants ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH131 Year 12 Structure synthesis and design
Chemical synthesis and design - Chemical synthesis involves the selection of particular reagents to form a product with specific properties (for example, pharmaceuticals, fuels, cosmetics, cleaning products) ACSPH039 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is conserved in the energy transfers and transformations that occur in an electrical circuit ACSPH042 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Power is the rate at which energy is transformed by a circuit component; power enables quantitative analysis of energy transformations in the circuit ACSPH043 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Resistance for ohmic and nonohmic components is defined as the ratio of potential difference across the component to the current in the component ACSPH060 Year 11 Linear Motion and Waves
Linear motion and force - Uniformly accelerated motion is described in terms of relationships between measurable scalar and vector quantities, including displacement, speed, velocity and acceleration ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH062 Year 11 Linear Motion and Waves
Linear motion and force - Vertical motion is analysed by assuming the acceleration due to gravity is constant near Earth’s surface ACSPH063 Year 11 Linear Motion and Waves
Linear motion and force - Newton’s Three Laws of Motion describe the relationship between the force or forces acting on an object, modelled as a point mass, and the motion of the object due to the application of the force or forces ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH066 Year 11 Linear Motion and Waves
Linear motion and force - Collisions may be elastic and inelastic; kinetic energy is conserved in elastic collisions ACSPH072 Year 11 Linear Motion and Waves
Waves - The superposition of waves in a medium may lead to the formation of standing waves and interference phenomena, including standing waves in pipes and on stretched strings ACSPH102 Year 12 Gravity and electromagnetism
Electromagnetism - Electrostatically charged objects exert a force upon one another; the magnitude of this force can be calculated using Coulomb’s Law ACSPH110 Year 12 Gravity and electromagnetism
Electromagnetism - A changing magnetic flux induces a potential difference; this process of electromagnetic induction is used in stepup and stepdown transformers, DC and AC generators, and AC induction motors ACSBL053 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Cellular respiration is a biochemical process that occurs in different locations in the cytosol and mitochondria and metabolises organic compounds, aerobically or anaerobically, to release useable energy in the form of ATP; the overall process can be repr ACSPH108 Year 12 Gravity and electromagnetism
Electromagnetism - Magnets, magnetic materials, moving charges and currentcarrying wires experience a force in a magnetic field; this force is utilised in DC electric motors ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system ACSPH100 Year 12 Gravity and electromagnetism
Gravity and motion - When an object experiences a net force of constant magnitude perpendicular to its velocity, it will undergo uniform circular motion, including circular motion on a horizontal plane and around a banked track ACSCH031 Year 11 Chemical fundamentals
Properties and structure of materials - The properties of ionic compounds (for example, high melting point, brittleness, ability to conduct electricity when liquid or in solution) are explained by modelling ionic bonding as ions arranged in a crystalline lattice structure with forces of attract ACSCH137 Year 12 Structure synthesis and design
Chemical synthesis and design - Fuels (for example, biodiesel, ethanol, hydrogen) can be synthesised from organic or inorganic sources using a range of chemical reactions including addition, oxidation and esterification ACSPH075 Year 11 Linear Motion and Waves
Waves - A ray model of light may be used to describe reflection, refraction and image formation from lenses and mirrors ACSPH077 Year 11 Linear Motion and Waves
Waves - The speed of light is finite and many orders of magnitude greater than the speed of mechanical waves (for example, sound and water waves); its intensity decreases in an inverse square relationship with distance from a point source ACSPH098 Year 12 Gravity and electromagnetism
Gravity and motion - The vector nature of the gravitational force can be used to analyse motion on inclined planes by considering the components of the gravitational force (that is, weight) parallel and perpendicular to the plane ACSPH103 Year 12 Gravity and electromagnetism
Electromagnetism - A positively charged body placed in an electric field will experience a force in the direction of the field; the strength of the electric field is defined as the force per unit charge ACSPH104 Year 12 Gravity and electromagnetism
Electromagnetism - Point charges and charged objects produce an electric field in the space that surrounds them; field theory attributes the electrostatic force on a point charge or charged body to the presence of an electric field ACSPH105 Year 12 Gravity and electromagnetism
Electromagnetism - When a charged body moves or is moved from one point to another in an electric field and its potential energy changes, work is done on or by the field
Products related to 'for'

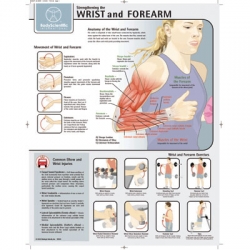
Strengthening the Wrist and Forearm Poster/Chart
STRENGTHENING THE WRIST AND FOREARM POSTER/CHART
The Strengthening the Wrist and Forearm Poster/Chart is one of the latest laminated posters/charts available for rehabilitation and fitness. Fitness Posters have been designed to show you the main areas of anatomy and which...
Order code: 7853-08

Vernier Centripetal Force Apparatus
No longer available.
VERNIER CENTRIPETAL FORCE APPARATUS Vernier's Centripetal Force Apparatus allows you to investigate the relationship between centripetal force, angular velocity, mass and radius. A force sensor measures the centripetal force exerted on a mass as it moves in a circle. A...
VERNIER CENTRIPETAL FORCE APPARATUS Vernier's Centripetal Force Apparatus allows you to investigate the relationship between centripetal force, angular velocity, mass and radius. A force sensor measures the centripetal force exerted on a mass as it moves in a circle. A...

Vernier Dual Range Force Sensor Replacement Parts Kit
VERNIER DUAL RANGE FORCE SENSOR REPLACEMENT PARTS KIT
The Dual-Range Force Sensor Replacement Parts Kit includes all of the small components for 10 Dual-Range Force Sensors.
Included:
• 10 thumb screws
• 10 rubber bumpers
• 10 hooks
• 10 nylon nuts...
Order code: DFS-RPK

IEC Dissectible Transformer Set
IEC DISSECTIBLE TRANSFORMER KIT
The IEC Dissectible Transformer can be assembled in many ways to produce a solenoid, magnet, choke, spot welder, induction heater and transformer.
The large iron core has a section of 35x35mm and the 'I' is clamped to the 'U' to close th...
Order code: EM1660-001
IEC Dissectible Transformer Small 12V AC 300+300+600T
IEC DISSECTIBLE TRANSFORMER SMALL 12V AC
The small IEC dissectible transformer is a cut-down version of the EM1973-001 Hodson Induction Kit. It consists of a 'U' and 'I' core and a set of coils for study of induction and transformers and it is designed to operate at a saf...
Order code: EM4089-001

Vernier Go Wireless 250mAh Battery (for Link)
VERNIER GO WIRELESS 250mAh BATTERY (FOR LINK)
The Go Wireless® 250 mAh Replacement Battery is a 250 mAh rechargeable, lithium-ion unit that comes with a one-year warranty. In typical use this battery will last for several years.
NOTE: This battery is used with t...
Order code: GW-BAT-250

IEC Light Box Collimating Lens and Slit Formers
IEC HODSON LIGHT BOX ONLY WITH COLLIMATING LENS & SLIT FORMERS
When the filters, shapes and mirrors of the full HL2060-001 IEC Hodson Light Box and Optical Set are not required, this basic version of the IEC 'Hodson' Light Box includes just the light box, collimating lens...
Order code: HL2060-020

IEC Light Box and Optical Set Hodson with Transformer
IEC HODSON LIGHT BOX AND OPTICAL SET WITH TRANSFORMER
This version of the HL2060-001 IEC Hodson Light Box and Optical Set is identical to the original except for the addition of a mini 240/12V transformer that allows the light box to be used with mains power. The transfor...
Order code: HL2061-001



Vernier Forensic Chemistry Experiments
VERNIER FORENSIC CHEMISTRY EXPERIMENTS
Engage students in learning fundamental chemistry concepts through Vernier's Forensic Chemistry Experiments lab manual.
Each of the 15 experiments in this book kicks off with a case study designed to engage high school and univer...
Order code: HSB-FCHEM

Vernier Forensic Chemistry Experiments Electronic
VERNIER FORENSIC CHEMISTRY EXPERIMENTS E-BOOK
Engage students in learning fundamental chemistry concepts through Vernier's Forensic Chemistry Experiments electronic lab manual.
Each of the 15 experiments in this book kicks off with a case study designed to engage high...
Order code: HSB-FCHEM-E

Plastomount Chick Showing Feather Down Just Before Hatching
Last one available
Chick, showing feather down just before hatching embedded in a clear plastic resin. Each plastomount has an embedded legend naming the individual specimens. Students often don't relate to imported specimens but they will recognise many of these specimens immediately.
Order code: K1356B
Slide Chick 18Hrs Formation of Notocord
Last one available
Chick, 18 hours, formation of notocord. A quality Australian made and prepared microscope slide.
Order code: K1902
Key Experiment Forensic Food Dye Tests
KECS9B: Forensic analysis of food dyes can be used to determine the mix of coloured substances in certain food drinks such as sports drinks.
Curriculum Topics
Chemistry: Properties and structure of materials
Chemistry: Properties of pure substances and mixtures...
Order code: KECS9B
IEC Transformer 240/12V 12A 3 Outputs
IEC TRANSFORMER 240/12V 12AMP x3 OUTPUTS
This simple but useful 220/240V to 12V AC transformer supplies 12V AC to three separate circuits up to a total load of 12A for any general laboratory purpose. The maximum of 12 amps may be drawn from any one pair of terminals or t...
Order code: LB4090-001

Beaker Borosilicate Glass Graduated 200ml Low Form
200ml graduated Borosilicate glass beaker.
Order code: LW0146-01
Beaker Borosilicate Glass Graduated 300ml Low Form
300ml graduated Borosilicate glass beaker.
Order code: LW0148-01

IEC Gyroscope Rotating Platform
Last few available so get in quick
IEC GYROSCOPE ROTATING PLATFORM
The IEC Gyroscope Rotating Platform is designed for students to safely stand on while using the MF1890-001 IEC Gyroscope. Students gain an engaging and exciting hands-on experience in rotational dynamics. As the gyroscopic forces react on t...
Order code: MF1891-001

IEC Dissectible Transformer Iron Core U/I Foot
IEC DISSECTIBLE TRANSFORMER U/I IRON CORE
A replacement Iron Core assembly as included in the EM1660-001 IEC Dissectible Transformer Kit. Complete with 'U' and 'I' with plastic foot to protect benches and special moulded clamp. The iron core section is 35x35mm.
Order code: PA1660-002

IEC Dissectible Transformer Coil Primary 800T
IEC DISSECTIBLE TRANSFORMER COIL PRIMARY 800T
A replacement 800T x 0.71mmD wire 240V AC Primary Coil to fit the 35x35mm U core included in the EM1660-001 IEC Dissectible Transformer Kit. Complete with removable mains cable and ON/OFF switch.
Order code: PA1660-003

IEC Dissectible Transformer Coil Secondary Low V Tapped
IEC DISSECTIBLE TRANSFORMER COIL SECONDARY LOW VOLT TAPPED
A replacement 20V tapped x1.4mmD wire Secondary Coil to fit the 35x35mm U core included in the EM1660-001 IEC Dissectible Transformer Kit.
Does not fit the old 40x40mm 'U' cores.
Order code: PA1660-004

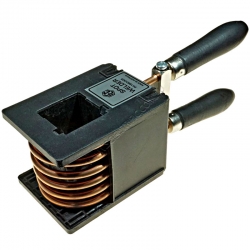
IEC Dissectible Transformer Coil Spot Welder
IEC DISSECTIBLE TRANSFORMER COIL SPOT WELDER
A replacement secondary coil spot welder wound around and enclosed inside a coil housing with handle for the EM1660-001 IEC Dissectible Transformer Kit.
Does not fit the old 40x40mm 'U' cores.
Order code: PA1660-005

IEC Dissectible Transformer Melting Ring
IEC DISSECTIBLE TRANSFORMER MELTING RING
A replacement copper melting ring with handle to melt solder or wax etc. to demonstrate induction heating with the EM1660-001 IEC Dissectible Transformer Kit.
Fits both 35x35mm and the old 40x40mm 'U' cores.
Order code: PA1660-006

IEC Dissectible Transformer Thompsons Ring
IEC DISSECTIBLE TRANSFORMER THOMPSON'S RING
A replacement aluminium Thompson's Ring to 'float' in the magnetic field and to demonstrate forces caused by induction with the EM1660-001 IEC Dissectible Transformer Kit.
Fits both 35x35mm and the old 40x40mm 'U' cores....
Order code: PA1660-007
386 results found for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16 | Next | View 100 per page


