424 low relevance results shown for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17 | Next | View 100 per page
Showing low relevance matches only. Return to normal search results
Forces and Moving - The way objects move depends on a variety of factors including their size and shape ACSSU031 Year 2 Chemical Sciences
Materials - Different materials can be combined, including by mixing, for a particular purpose ACSSU033 Year 2 Physical Sciences
Forces and Moving - A push or a pull affects how an object moves or changes shape ACSSU076 Year 4 Physical Sciences
Forces and Moving - Forces can be exerted by one object on another through direct contact or from a distance ACSSU080 Year 5 Physical Sciences
Light and Sound - Light from a source forms shadows and can be absorbed, reflected and refracted ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU117 Year 7 Physical Sciences
Forces and Machines - Change to an object’s motion is caused by unbalanced forces, including Earth’s gravitational attraction, acting on the object ACSSU153 Year 8 Earth and Space Sciences
Rocks and Minerals - Sedimentary, igneous and metamorphic rocks contain minerals and are formed by processes that occur within Earth over a variety of timescales ACSSU155 Year 8 Physical Sciences
Energy Forms - Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems ACSSU178 Year 9 Chemical Sciences
Chemical Reactions - Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created or destroyed ACSSU225 Year 8 Chemical Sciences
Chemical Reactions - Chemical change involves substances reacting to form new substances ACSSU190 Year 10 Physical Sciences
Energy Conservation - Energy conservation in a system can be explained by describing energy transfers and transformations ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL115 Year 12 Maintaining the internal environment
Homeostasis - Animals, whether osmo-regulators or osmo-conformers, and plants, have various mechanisms to maintain water balance that involve structural features, and behavioural, physiological and homeostatic responses ACSCH018 Year 11 Chemical fundamentals
Properties and structure of atoms - Atoms can be modelled as a nucleus surrounded by electrons in distinct energy levels, held together by electrostatic forces of attraction between the nucleus and electrons; atoms can be represented using electron shell diagrams (all electron shells or val ACSCH025 Year 11 Chemical fundamentals
Properties and structure of materials - Materials are either pure substances with distinct measurable properties (for example, melting and boiling point, reactivity, strength, density) or mixtures with properties dependent on the identity and relative amounts of the substances that make up the ACSCH030 Year 11 Chemical fundamentals
Properties and structure of materials - Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons and protons; ions are represented by formulae which include the number of constituent atoms and the charge of the ion (for example, O2–, SO42–) ACSCH032 Year 11 Chemical fundamentals
Properties and structure of materials - The characteristic properties of metals (for example, malleability, thermal conductivity, electrical conductivity) are explained by modelling metallic bonding as a regular arrangement of positive ions (cations) made stable by electrostatic forces of attra ACSCH036 Year 11 Chemical fundamentals
Chemical reactions - All chemical reactions involve the creation of new substances and associated energy transformations, commonly observable as changes in the temperature of the surroundings and/or the emission of light ACSCH037 Year 11 Chemical fundamentals
Chemical reactions - Endothermic and exothermic reactions can be explained in terms of the Law of Conservation of Energy and the breaking and reforming of bonds; heat energy released or absorbed can be represented in thermochemical equations ACSCH039 Year 11 Chemical fundamentals
Chemical reactions - A mole is a precisely defined quantity of matter equal to Avogadro’s number of particles; the mole concept and the Law of Conservation of Mass can be used to calculate the mass of reactants and products in a chemical reaction ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH059 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - Data from chromatography techniques (for example, thin layer, gas and highperformance liquid chromatography) can be used to determine the composition and purity of substances; the separation of the components is caused by the variation of strength of the ACSCH060 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The behaviour of gases, including the qualitative relationships between pressure, temperature and volume, can be explained using kinetic theory ACSCH065 Year 11 Molecular interactions and reactions
Aqueous solutions and acidity - The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature ACSCH069 Year 11 Molecular interactions and reactions
Rates of chemical reactions - The rate of chemical reactions can be quantified by measuring the rate of formation of products or the depletion of reactants ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH131 Year 12 Structure synthesis and design
Chemical synthesis and design - Chemical synthesis involves the selection of particular reagents to form a product with specific properties (for example, pharmaceuticals, fuels, cosmetics, cleaning products) ACSPH039 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is conserved in the energy transfers and transformations that occur in an electrical circuit ACSPH042 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Power is the rate at which energy is transformed by a circuit component; power enables quantitative analysis of energy transformations in the circuit ACSPH043 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Resistance for ohmic and nonohmic components is defined as the ratio of potential difference across the component to the current in the component ACSPH060 Year 11 Linear Motion and Waves
Linear motion and force - Uniformly accelerated motion is described in terms of relationships between measurable scalar and vector quantities, including displacement, speed, velocity and acceleration ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH062 Year 11 Linear Motion and Waves
Linear motion and force - Vertical motion is analysed by assuming the acceleration due to gravity is constant near Earth’s surface ACSPH063 Year 11 Linear Motion and Waves
Linear motion and force - Newton’s Three Laws of Motion describe the relationship between the force or forces acting on an object, modelled as a point mass, and the motion of the object due to the application of the force or forces ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH066 Year 11 Linear Motion and Waves
Linear motion and force - Collisions may be elastic and inelastic; kinetic energy is conserved in elastic collisions ACSPH072 Year 11 Linear Motion and Waves
Waves - The superposition of waves in a medium may lead to the formation of standing waves and interference phenomena, including standing waves in pipes and on stretched strings ACSPH102 Year 12 Gravity and electromagnetism
Electromagnetism - Electrostatically charged objects exert a force upon one another; the magnitude of this force can be calculated using Coulomb’s Law ACSPH110 Year 12 Gravity and electromagnetism
Electromagnetism - A changing magnetic flux induces a potential difference; this process of electromagnetic induction is used in stepup and stepdown transformers, DC and AC generators, and AC induction motors ACSBL053 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Cellular respiration is a biochemical process that occurs in different locations in the cytosol and mitochondria and metabolises organic compounds, aerobically or anaerobically, to release useable energy in the form of ATP; the overall process can be repr ACSPH108 Year 12 Gravity and electromagnetism
Electromagnetism - Magnets, magnetic materials, moving charges and currentcarrying wires experience a force in a magnetic field; this force is utilised in DC electric motors ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system ACSPH100 Year 12 Gravity and electromagnetism
Gravity and motion - When an object experiences a net force of constant magnitude perpendicular to its velocity, it will undergo uniform circular motion, including circular motion on a horizontal plane and around a banked track ACSCH031 Year 11 Chemical fundamentals
Properties and structure of materials - The properties of ionic compounds (for example, high melting point, brittleness, ability to conduct electricity when liquid or in solution) are explained by modelling ionic bonding as ions arranged in a crystalline lattice structure with forces of attract ACSCH137 Year 12 Structure synthesis and design
Chemical synthesis and design - Fuels (for example, biodiesel, ethanol, hydrogen) can be synthesised from organic or inorganic sources using a range of chemical reactions including addition, oxidation and esterification ACSPH075 Year 11 Linear Motion and Waves
Waves - A ray model of light may be used to describe reflection, refraction and image formation from lenses and mirrors ACSPH077 Year 11 Linear Motion and Waves
Waves - The speed of light is finite and many orders of magnitude greater than the speed of mechanical waves (for example, sound and water waves); its intensity decreases in an inverse square relationship with distance from a point source ACSPH098 Year 12 Gravity and electromagnetism
Gravity and motion - The vector nature of the gravitational force can be used to analyse motion on inclined planes by considering the components of the gravitational force (that is, weight) parallel and perpendicular to the plane ACSPH103 Year 12 Gravity and electromagnetism
Electromagnetism - A positively charged body placed in an electric field will experience a force in the direction of the field; the strength of the electric field is defined as the force per unit charge ACSPH104 Year 12 Gravity and electromagnetism
Electromagnetism - Point charges and charged objects produce an electric field in the space that surrounds them; field theory attributes the electrostatic force on a point charge or charged body to the presence of an electric field ACSPH105 Year 12 Gravity and electromagnetism
Electromagnetism - When a charged body moves or is moved from one point to another in an electric field and its potential energy changes, work is done on or by the field

















424 low relevance results shown for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17 | Next | View 100 per page
Showing low relevance matches only. Return to normal search results
Curriculum resources related to 'for'
ACSSU005 Foundation Physical SciencesForces and Moving - The way objects move depends on a variety of factors including their size and shape ACSSU031 Year 2 Chemical Sciences
Materials - Different materials can be combined, including by mixing, for a particular purpose ACSSU033 Year 2 Physical Sciences
Forces and Moving - A push or a pull affects how an object moves or changes shape ACSSU076 Year 4 Physical Sciences
Forces and Moving - Forces can be exerted by one object on another through direct contact or from a distance ACSSU080 Year 5 Physical Sciences
Light and Sound - Light from a source forms shadows and can be absorbed, reflected and refracted ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU117 Year 7 Physical Sciences
Forces and Machines - Change to an object’s motion is caused by unbalanced forces, including Earth’s gravitational attraction, acting on the object ACSSU153 Year 8 Earth and Space Sciences
Rocks and Minerals - Sedimentary, igneous and metamorphic rocks contain minerals and are formed by processes that occur within Earth over a variety of timescales ACSSU155 Year 8 Physical Sciences
Energy Forms - Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems ACSSU178 Year 9 Chemical Sciences
Chemical Reactions - Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created or destroyed ACSSU225 Year 8 Chemical Sciences
Chemical Reactions - Chemical change involves substances reacting to form new substances ACSSU190 Year 10 Physical Sciences
Energy Conservation - Energy conservation in a system can be explained by describing energy transfers and transformations ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL115 Year 12 Maintaining the internal environment
Homeostasis - Animals, whether osmo-regulators or osmo-conformers, and plants, have various mechanisms to maintain water balance that involve structural features, and behavioural, physiological and homeostatic responses ACSCH018 Year 11 Chemical fundamentals
Properties and structure of atoms - Atoms can be modelled as a nucleus surrounded by electrons in distinct energy levels, held together by electrostatic forces of attraction between the nucleus and electrons; atoms can be represented using electron shell diagrams (all electron shells or val ACSCH025 Year 11 Chemical fundamentals
Properties and structure of materials - Materials are either pure substances with distinct measurable properties (for example, melting and boiling point, reactivity, strength, density) or mixtures with properties dependent on the identity and relative amounts of the substances that make up the ACSCH030 Year 11 Chemical fundamentals
Properties and structure of materials - Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons and protons; ions are represented by formulae which include the number of constituent atoms and the charge of the ion (for example, O2–, SO42–) ACSCH032 Year 11 Chemical fundamentals
Properties and structure of materials - The characteristic properties of metals (for example, malleability, thermal conductivity, electrical conductivity) are explained by modelling metallic bonding as a regular arrangement of positive ions (cations) made stable by electrostatic forces of attra ACSCH036 Year 11 Chemical fundamentals
Chemical reactions - All chemical reactions involve the creation of new substances and associated energy transformations, commonly observable as changes in the temperature of the surroundings and/or the emission of light ACSCH037 Year 11 Chemical fundamentals
Chemical reactions - Endothermic and exothermic reactions can be explained in terms of the Law of Conservation of Energy and the breaking and reforming of bonds; heat energy released or absorbed can be represented in thermochemical equations ACSCH039 Year 11 Chemical fundamentals
Chemical reactions - A mole is a precisely defined quantity of matter equal to Avogadro’s number of particles; the mole concept and the Law of Conservation of Mass can be used to calculate the mass of reactants and products in a chemical reaction ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH059 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - Data from chromatography techniques (for example, thin layer, gas and highperformance liquid chromatography) can be used to determine the composition and purity of substances; the separation of the components is caused by the variation of strength of the ACSCH060 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The behaviour of gases, including the qualitative relationships between pressure, temperature and volume, can be explained using kinetic theory ACSCH065 Year 11 Molecular interactions and reactions
Aqueous solutions and acidity - The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature ACSCH069 Year 11 Molecular interactions and reactions
Rates of chemical reactions - The rate of chemical reactions can be quantified by measuring the rate of formation of products or the depletion of reactants ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH131 Year 12 Structure synthesis and design
Chemical synthesis and design - Chemical synthesis involves the selection of particular reagents to form a product with specific properties (for example, pharmaceuticals, fuels, cosmetics, cleaning products) ACSPH039 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is conserved in the energy transfers and transformations that occur in an electrical circuit ACSPH042 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Power is the rate at which energy is transformed by a circuit component; power enables quantitative analysis of energy transformations in the circuit ACSPH043 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Resistance for ohmic and nonohmic components is defined as the ratio of potential difference across the component to the current in the component ACSPH060 Year 11 Linear Motion and Waves
Linear motion and force - Uniformly accelerated motion is described in terms of relationships between measurable scalar and vector quantities, including displacement, speed, velocity and acceleration ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH062 Year 11 Linear Motion and Waves
Linear motion and force - Vertical motion is analysed by assuming the acceleration due to gravity is constant near Earth’s surface ACSPH063 Year 11 Linear Motion and Waves
Linear motion and force - Newton’s Three Laws of Motion describe the relationship between the force or forces acting on an object, modelled as a point mass, and the motion of the object due to the application of the force or forces ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH066 Year 11 Linear Motion and Waves
Linear motion and force - Collisions may be elastic and inelastic; kinetic energy is conserved in elastic collisions ACSPH072 Year 11 Linear Motion and Waves
Waves - The superposition of waves in a medium may lead to the formation of standing waves and interference phenomena, including standing waves in pipes and on stretched strings ACSPH102 Year 12 Gravity and electromagnetism
Electromagnetism - Electrostatically charged objects exert a force upon one another; the magnitude of this force can be calculated using Coulomb’s Law ACSPH110 Year 12 Gravity and electromagnetism
Electromagnetism - A changing magnetic flux induces a potential difference; this process of electromagnetic induction is used in stepup and stepdown transformers, DC and AC generators, and AC induction motors ACSBL053 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Cellular respiration is a biochemical process that occurs in different locations in the cytosol and mitochondria and metabolises organic compounds, aerobically or anaerobically, to release useable energy in the form of ATP; the overall process can be repr ACSPH108 Year 12 Gravity and electromagnetism
Electromagnetism - Magnets, magnetic materials, moving charges and currentcarrying wires experience a force in a magnetic field; this force is utilised in DC electric motors ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system ACSPH100 Year 12 Gravity and electromagnetism
Gravity and motion - When an object experiences a net force of constant magnitude perpendicular to its velocity, it will undergo uniform circular motion, including circular motion on a horizontal plane and around a banked track ACSCH031 Year 11 Chemical fundamentals
Properties and structure of materials - The properties of ionic compounds (for example, high melting point, brittleness, ability to conduct electricity when liquid or in solution) are explained by modelling ionic bonding as ions arranged in a crystalline lattice structure with forces of attract ACSCH137 Year 12 Structure synthesis and design
Chemical synthesis and design - Fuels (for example, biodiesel, ethanol, hydrogen) can be synthesised from organic or inorganic sources using a range of chemical reactions including addition, oxidation and esterification ACSPH075 Year 11 Linear Motion and Waves
Waves - A ray model of light may be used to describe reflection, refraction and image formation from lenses and mirrors ACSPH077 Year 11 Linear Motion and Waves
Waves - The speed of light is finite and many orders of magnitude greater than the speed of mechanical waves (for example, sound and water waves); its intensity decreases in an inverse square relationship with distance from a point source ACSPH098 Year 12 Gravity and electromagnetism
Gravity and motion - The vector nature of the gravitational force can be used to analyse motion on inclined planes by considering the components of the gravitational force (that is, weight) parallel and perpendicular to the plane ACSPH103 Year 12 Gravity and electromagnetism
Electromagnetism - A positively charged body placed in an electric field will experience a force in the direction of the field; the strength of the electric field is defined as the force per unit charge ACSPH104 Year 12 Gravity and electromagnetism
Electromagnetism - Point charges and charged objects produce an electric field in the space that surrounds them; field theory attributes the electrostatic force on a point charge or charged body to the presence of an electric field ACSPH105 Year 12 Gravity and electromagnetism
Electromagnetism - When a charged body moves or is moved from one point to another in an electric field and its potential energy changes, work is done on or by the field
Products related to 'for'

Diffraction Grating 300 lines/mm
A quality glass diffraction grating from the UK for hands on-experiments in physics and chemistry classes.
Slide size: 50x50mm
Aperture size: 36x24mm
300 lines per mm.
Order code: SC3607

ScienceWiz Teacher 6 Pack Chemistry
Candle making, squirting water, freezing, thawing, glop and mud pie experiments -- things every student should do to explore the matter of matter. This ScienceWiz™ kit deliberately relates and explains favourite childhood activities to chemistry. The importance of this to lat...
Order code: SCW9904
ScienceWiz Chemistry Kit Sample
Candle making, squirting water, freezing, thawing, glop and mud pie experiments -- things every student should do to explore the matter of matter. This ScienceWiz™ kit deliberately relates and explains favourite childhood activities to chemistry. The importance of this to lat...
Order code: SCW9904-S

Diffraction Grating 600 lines/mm
A quality glass diffraction grating from the UK for hands on-experiments in physics and chemistry classes.
Slide size: 50x50mm
Aperture size: 36x24mm
600 lines per mm.
Order code: SC3608

Diffraction Grating 1200 lines/mm
A quality glass diffraction grating from the UK for hands on-experiments in physics and chemistry classes.
Slide size: 50x50mm
Aperture size: 36x24mm
1200 lines per mm
Order code: SC3609

ScienceWiz Teacher 6 Pack Chemistry Plus
An exciting set of selected experiments designed to make elements elementary and explore the periodicity of the periodic table. Learn about atoms and the stuff they are made of: protons, neutrons and electrons. The beautifully illustrated book and materials kit takes students thr...
Order code: SCW9910
ScienceWiz Chemistry Plus Kit Sample
An exciting set of selected experiments designed to make elements elementary and explore the periodicity of the periodic table. Learn about atoms and the stuff they are made of: protons, neutrons and electrons. The beautifully illustrated book and materials kit takes students thr...
Order code: SCW9910-S

ScienceWiz Teacher 6 Pack Physics
This ScienceWiz™ kit introduces the concepts of inertia, the laws of motion, mass, force and weight, velocity and acceleration, circular motion and centripetal force. Concepts are explored with time tested experiments and highly visual illustrations.
This Teacher's Pack inc...
Order code: SCW9912
ScienceWiz Physics Kit Sample
This ScienceWiz™ kit introduces the concepts of inertia, the laws of motion, mass, force and weight, velocity and acceleration, circular motion and centripetal force. Concepts are explored with time tested experiments and highly visual illustrations.
This sample kit include...
Order code: SCW9912-S

ScienceWiz Teacher 6 Pack Energy
Discover what energy is, how we make it now and the choices awaiting us in the future. Learn about carbon emissions and climate change. This ScienceWiz™ Teacher's Pack includes enough equipment for 6 students to explore and develop a fundamental understanding of the key concep...
Order code: SCW9905
ScienceWiz Energy Kit Sample
Discover what energy is, how we make it now and the choices awaiting us in the future. Learn about carbon emissions and climate change. This ScienceWiz™ kit enables a student to explore and develop a fundamental understanding of the key concepts of energy while building a foun...
Order code: SCW9905-S

Molymod Introductory Student Set 48 Atoms
MOLYMOD INTRODUCTORY STUDENT SET
Supplied in a lidded 4-compartment sturdy plastic box, this student introductory Molymod® set contains a total of 48 atoms plus links and a link remover tool. Instructions are included for building over 30 molecules.
A quality, genuine...
Order code: MMS-001

ScienceWiz Teacher 6 Pack DNA
Make the DNA revolution accessible! Central concepts in molecular biology become child's play in this cutting edge science kit. Discoveries in molecular biology are among the most important scientific advancements of our time.
This ScienceWiz™ Teacher's Pack includes enough...
Order code: SCW9911
ScienceWiz DNA Kit Sample
Make the DNA revolution accessible! Central concepts in molecular biology become child's play in this cutting edge science kit. Discoveries in molecular biology are among the most important scientific advancements of our time.
This sample kit enables a student to explore and d...
Order code: SCW9911-S

ScienceWiz Teacher 6 Pack Inventions
The ScienceWiz™ Inventions kit allows students to build models of inventions made with coils from the 19th and early 20th century to explore basic electronic components and the fundamentals of electromagnetism. Step-by-step 3D directions and the use of everyday materials bring...
Order code: SCW9903
ScienceWiz Inventions Kit Sample
The ScienceWiz™ Inventions kit allows students to build models of inventions made with coils from the 19th and early 20th century and to explore basic electronic components and the fundamentals of electromagnetism. Step-by-step 3D directions and the use of everyday materials b...
Order code: SCW9903-S

Molymod Organic Teachers Set - 111 Atoms
MOLYMOD ORGANIC CHEMISTRY TEACHERS SET
Supplied in a lidded large-compartment, sturdy plastic box, this organic chemistry Molymod® set contains a total of 111 atoms plus links.
Instructions are included for building over 30 molecules.
A quality, genuine Molymod® at...
Order code: MMS-003

Molymod Organic/Inorganic Teachers Set - 108 Atoms
A genuine Molymod® product NOT a cloned look-a-like.
Supplied in a sturdy plastic box with product information and instructions for building over 30 molecules. Contains a total of 106 'atoms' plus links as listed below.
Atoms - 108:
14 x Carbon, 4-holes tetra...
Order code: MMS-004

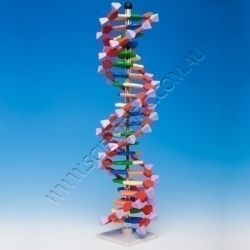
Molymod DNA Model Kit 12 Layer
MOLYMOD DNA 12 LAYER MODEL KIT
This advanced model of one complete turn of the DNA double helix is designed for self-assembly of the 12 layers. The double helix structure becomes evident as the model is constructed.
Components include colour coded bases (G,C,A and T)...
Order code: AMDNA-060-12

Molymod DNA Model Kit 22 Layer
MOLYMOD DNA 22 LAYER MODEL KIT
This advanced model of two complete turns of the DNA double helix is designed for self-assembly of the 22 layers. The double helix structure becomes evident as the model is constructed.
The components include colour coded bases (G, C, A a...
Order code: AMDNA-060-22

ScienceWiz Crazy Chemistry Party Kit
Crazy + Chemistry + Party + 7 Kids + Adult Supervision ==> An explosively good time!
Why not make science a celebration?
Crazy Chemistry Party consists of several projects selected from those in the ScienceWiz™ kits designed by Dr. Penny Norman (Ph.D. from UC...
Order code: SCW9022

ScienceWiz Chess: Once a Pawn a Time
Want your students to love chess as well as master it?
In ScienceWiz Chess: Once A Pawn A Time™ author and teacher Patzi Stewart takes a beginner through the fundamentals of playing chess in a new and engaging way.
Instructional Storybook One begins with a whimsical intr...
Order code: SCW7851

ScienceWiz Pohaku Dominos
A three colour, four sided domino game with only one rule -- match the colours.
Four game variations keep play interesting for young and old alike. And also challenge those who find maths intriguing.
Pohaku™ Dominos can be played with pieces hidden as a game of chance ...
Order code: SCW7852

Horizon Bio-Energy Discovery Kit
HORIZON BIO-ENERGY DISCOVERY KIT
Demonstrates the latest in fuel cell technology using ethanol as a fuel. The fuel cell kit directly converts chemical energy in ethanol (alcohol) to electrical energy without combustion. The device can run non-stop for hours, providing an ...
Order code: FCJJ-22
424 low relevance results shown for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17 | Next | View 100 per page



 ,
,