28 results found for 'acids'. |1|2 | Next | View 100 per page Strip plurals: acids to acid
Low relevance matches: 9 other results may be of interest to you. Show low relevance matches
Chemical Reactions - Chemical reactions, including combustion and the reactions of acids, are important in both non-living and living systems and involve energy transfer ACSCH091 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Over time, physical changes and reversible chemical reactions reach a state of dynamic equilibrium in a closed system, with the relative concentrations of products and reactants defining the position of equilibrium ACSCH096 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Equilibrium position can be predicted qualitatively using equilibrium constants ACSCH097 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acids are substances that can act as proton (hydrogen ion) donors and can be classified as monoprotic or polyprotic depending on the number of protons donated by each molecule of the acid ACSCH098 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The strength of acids is explained by the degree of ionisation at equilibrium in aqueous solution, which can be represented with chemical equations and equilibrium constants (Ka) ACSCH099 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The relationship between acids and bases in equilibrium systems can be explained using the Brønsted Lowry model and represented using chemical equations that illustrate the transfer of hydrogen ions ACSCH100 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The pH scale is a logarithmic scale and the pH of a solution can be calculated from the concentration of hydrogen ions; Kw can be used to calculate the concentration of hydrogen ions from the concentration of hydroxide ions in a solution ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH102 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Volumetric analysis methods involving acidbase reactions rely on the identification of an equivalence point by measuring the associated change in pH, using chemical indicators or pH meters, to reveal an observable end point ACSCH103 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - A range of reactions, including displacement reactions of metals, combustion, corrosion, and electrochemical processes, can be modelled as redox reactions involving oxidation of one substance and reduction of another substance ACSCH104 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Oxidation can be modelled as the loss of electrons from a chemical species, and reduction can be modelled as the gain of electrons by a chemical species; these processes can be represented using half equations ACSCH106 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - The relative strength of oxidising and reducing agents can be determined by comparing standard electrode potentials ACSCH107 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Electrochemical cells, including galvanic and electrolytic cells, consist of oxidation and reduction half reactions connected via an external circuit that allows electrons to move from the anode (oxidation reaction) to the cathode (reduction reaction) ACSCH108 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Galvanic cells, including fuel cells, generate an electrical potential difference from a spontaneous redox reaction; they can be represented as cell diagrams including anode and cathode halfequations ACSCH110 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Cell potentials at standard conditions can be calculated from standard electrode potentials; these values can be used to compare cells constructed from different materials



















28 results found for 'acids'. |1|2 | Next | View 100 per page
Low relevance matches: 9 other results may be of interest to you. Show low relevance matches
Curriculum resources related to 'acids'
ACSSU179 Year 9 Chemical SciencesChemical Reactions - Chemical reactions, including combustion and the reactions of acids, are important in both non-living and living systems and involve energy transfer ACSCH091 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Over time, physical changes and reversible chemical reactions reach a state of dynamic equilibrium in a closed system, with the relative concentrations of products and reactants defining the position of equilibrium ACSCH096 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Equilibrium position can be predicted qualitatively using equilibrium constants ACSCH097 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acids are substances that can act as proton (hydrogen ion) donors and can be classified as monoprotic or polyprotic depending on the number of protons donated by each molecule of the acid ACSCH098 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The strength of acids is explained by the degree of ionisation at equilibrium in aqueous solution, which can be represented with chemical equations and equilibrium constants (Ka) ACSCH099 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The relationship between acids and bases in equilibrium systems can be explained using the Brønsted Lowry model and represented using chemical equations that illustrate the transfer of hydrogen ions ACSCH100 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The pH scale is a logarithmic scale and the pH of a solution can be calculated from the concentration of hydrogen ions; Kw can be used to calculate the concentration of hydrogen ions from the concentration of hydroxide ions in a solution ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH102 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Volumetric analysis methods involving acidbase reactions rely on the identification of an equivalence point by measuring the associated change in pH, using chemical indicators or pH meters, to reveal an observable end point ACSCH103 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - A range of reactions, including displacement reactions of metals, combustion, corrosion, and electrochemical processes, can be modelled as redox reactions involving oxidation of one substance and reduction of another substance ACSCH104 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Oxidation can be modelled as the loss of electrons from a chemical species, and reduction can be modelled as the gain of electrons by a chemical species; these processes can be represented using half equations ACSCH106 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - The relative strength of oxidising and reducing agents can be determined by comparing standard electrode potentials ACSCH107 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Electrochemical cells, including galvanic and electrolytic cells, consist of oxidation and reduction half reactions connected via an external circuit that allows electrons to move from the anode (oxidation reaction) to the cathode (reduction reaction) ACSCH108 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Galvanic cells, including fuel cells, generate an electrical potential difference from a spontaneous redox reaction; they can be represented as cell diagrams including anode and cathode halfequations ACSCH110 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Cell potentials at standard conditions can be calculated from standard electrode potentials; these values can be used to compare cells constructed from different materials
Products related to 'acids'

Molymod Amino Acid Set of Seven Models
MOLYMOD AMINO ACID 7 MODEL SET
Use this set of genuine Molymod® atoms to build a model of each of 7 L-configuration amino acids.
Includes a total of 125 atom parts (23mm red oxygen, 23mm blue nitrogen, 23mm black carbon, 23mm yellow sulphur and 19mm white dome hydro...
Order code: MKA-120-7

Molymod Amino Acid Set of 20 Models
MOLYMOD AMINO ACID 20 MODEL SET
Use this set of genuine Molymod® atoms to build a model of each of the 20 common amino acids.
Includes a total of 385 atom parts (23mm red oxygen, 23mm blue nitrogen, 23mm black carbon and 19mm white dome hydrogen) with links (short whi...
Order code: MKA-121-20

Molymod Saturated Fatty Acid Stearic Acid
MOLYMOD STEARIC ACID
A set of genuine Molymod® black carbon, red oxygen and white dome hydrogen atoms,
plus links and a tool to build a compact molecule of Stearic Acid C18H36O2
which is one example of a saturated fatty acid.
The...
Order code: MKS-117-S

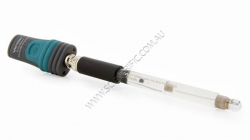
Vernier Go Direct pH Sensor
VERNIER GO DIRECT pH SENSOR
Vernier's Go Direct pH Sensor is a general purpose pH sensor used to monitor the pH of aqueous solutions. It directly connects wirelessly via Bluetooth® or wired via USB to your platform.
The Vernier Go Direct pH Sensor is an important and v...
Order code: GDX-PH



Vernier Go Direct Polarimeter
VERNIER GO DIRECT POLARIMETER
The concept of chirality can be difficult for students to visualize. Vernier's Go Direct Polarimeter provides a visual representation of this concept by measuring the optical rotation of optical isomers such as sugars, amino acids and protein...
Order code: GDX-POL

Vernier Stainless Steel Temperature Probe
VERNIER STAINLESS STEEL TEMPERATURE PROBE
Vernier's Stainless Steel Temperature Probe is a rugged, general-purpose temperature sensor with a sealed stainless steel shaft and tip that can be used in organic liquids, salt solutions, acids and bases. Use it as you would use ...
Order code: TMP-BTA



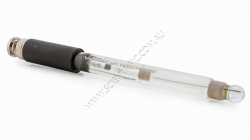
Vernier pH Sensor
VERNIER PH SENSOR
Vernier's general purpose pH sensor can be used across disciplines including chemistry, biology, middle school science and environmental science.
Use the pH Sensor just as you would a traditional pH meter with the additional advantages of automated da...
Order code: PH-BTA



Vernier Go Direct Glass-Body pH Sensor
VERNIER GO DIRECT GLASS BODY pH SENSOR
This high-quality glass body pH sensor can be used in non-aqueous solutions and solutions that contain organic solvents, strong acids or strong bases. The electrode features a sealed, gel-filled Ag-AgCl combination reference electrod...
Order code: GDX-GPH



Vernier Go Direct Platinum-Cell Conductivity Probe
VERNIER GO DIRECT PLATINUM-CELL CONDUCTIVITY PROBE
Perfect for senior secondary and university general chemistry, Vernier's Go Direct Platinum-Cell Conductivity Probe provides an accurate and easy measurement of a solution’s conductivity or its total ion concentration. Th...
Order code: GDX-CONPT

Vernier Go Direct pH Teacher Pack
VERNIER GO DIRECT pH SENSOR TEACHER PACK
Buy more and save. With Vernier's Go Direct pH Teacher Pack you receive eight Go Direct pH Sensors and the convenient Vernier Go Direct Charging Station that allows you to charge eight Vernier Go Direct pH Sensors at a time.
The...
Order code: GDX-PH-TP



Vernier Go Direct Glass Body pH BNC Electrode
VERNIER GO DIRECT GLASS BODY pH BNC ELECTRODE
Connect the Glass-Body pH BNC Electrode to Vernier's Go Direct™ Electrode Amplifier to measure pH in aqueous, heterogeneous and organic solutions.
This high-quality glass body pH electrode can be used in non-aqueous solutio...
Order code: GDX-GPH-BNC



Vernier Wide Range Temperature Probe
VERNIER WIDE RANGE TEMPERATURE PROBE
Vernier's rugged Wide-Range Temperature Probe measures a wide temperature range from –20 to 330°C. The high upper limit of the sensor allows melting point determinations of most organic compounds. It uses Resistance Temperature Detecti...
Order code: WRT-BTA

Biology with Vernier
BIOLOGY WITH VERNIER
Biology with Vernier is a lab book containing 31 experiments in cell respiration, photosynthesis, membrane diffusion, osmosis, human physiology, transpiration, fermentation and other important biology concepts.
Experiments are included for 12 Vernier...
Order code: BWV

Biology with Vernier - Electronic Version
BIOLOGY WITH VERNIER - ELECTRONIC
Biology with Vernier is a lab book containing 31 experiments in cell respiration, photosynthesis, membrane diffusion, osmosis, human physiology, transpiration, fermentation and other important biology concepts.
Experiments are included f...
Order code: BWV-E

Chemistry with Vernier
CHEMISTRY WITH VERNIER
Chemistry with Vernier is a lab book containing more than 35 experiments in thermochemistry, gas laws, acid-base reactions, equilibrium, electrochemistry, electrolytes, states of matter and more.
Experiments are included for the Gas Pressure Sen...
Order code: CWV

Chemistry with Vernier - Electronic Version
CHEMISTRY WITH VERNIER - ELECTRONIC
Chemistry with Vernier is a lab book containing more than 35 experiments in thermochemistry, gas laws, acid-base reactions, equilibrium, electrochemistry, electrolytes, states of matter and more.
Experiments are included for the Gas...
Order code: CWV-E

Physical Science with Vernier
PHYSICAL SCIENCE WITH VERNIER
Physical Science with Vernier is a lab book containing 40 ready-to-use experiments for physical science in grades 6-10 that are perfect for introductory physical science and integrated science classes. Experiments are included for 12 Vernier ...
Order code: PSV

Physical Science with Vernier - Electronic Version
PHYSICAL SCIENCE WITH VERNIER
Physical Science with Vernier is a lab book containing 40 ready-to-use experiments for physical science in grades 6-10 that are perfect for introductory physical science and integrated science classes. Experiments are included for 12 Vernier ...
Order code: PSV-E

Agricultural Science with Vernier - Electronic Version
AGRICULTURAL SCIENCE WITH VERNIER - ELECTRONIC
Agricultural Science with Vernier is a digital lab book containing a collection of 29 experiments that can be useful in teaching agricultural science at the high school or college level. Some experiments are designed to be do...
Order code: AWV-E

ScienceWiz Teacher 6 Pack Chemistry Plus
An exciting set of selected experiments designed to make elements elementary and explore the periodicity of the periodic table. Learn about atoms and the stuff they are made of: protons, neutrons and electrons. The beautifully illustrated book and materials kit takes students thr...
Order code: SCW9910


ScienceWiz Chemistry Plus Kit Sample
An exciting set of selected experiments designed to make elements elementary and explore the periodicity of the periodic table. Learn about atoms and the stuff they are made of: protons, neutrons and electrons. The beautifully illustrated book and materials kit takes students thr...
Order code: SCW9910-S



Electro Chem Clock
ELECTRO CHEM CLOCK
Explore the exciting science of electrochemistry with this fun electric lab. Build a battery that produces electricity from lemon juice. Use the battery to power a digital clock with LCD display. Experiment with using other acids such as cola or even sa...
Order code: 659073
28 results found for 'acids'. |1|2 | Next | View 100 per page



