424 low relevance results shown for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17 | Next | View 100 per page
Showing low relevance matches only. Return to normal search results
Forces and Moving - The way objects move depends on a variety of factors including their size and shape ACSSU031 Year 2 Chemical Sciences
Materials - Different materials can be combined, including by mixing, for a particular purpose ACSSU033 Year 2 Physical Sciences
Forces and Moving - A push or a pull affects how an object moves or changes shape ACSSU076 Year 4 Physical Sciences
Forces and Moving - Forces can be exerted by one object on another through direct contact or from a distance ACSSU080 Year 5 Physical Sciences
Light and Sound - Light from a source forms shadows and can be absorbed, reflected and refracted ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU117 Year 7 Physical Sciences
Forces and Machines - Change to an object’s motion is caused by unbalanced forces, including Earth’s gravitational attraction, acting on the object ACSSU153 Year 8 Earth and Space Sciences
Rocks and Minerals - Sedimentary, igneous and metamorphic rocks contain minerals and are formed by processes that occur within Earth over a variety of timescales ACSSU155 Year 8 Physical Sciences
Energy Forms - Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems ACSSU178 Year 9 Chemical Sciences
Chemical Reactions - Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created or destroyed ACSSU225 Year 8 Chemical Sciences
Chemical Reactions - Chemical change involves substances reacting to form new substances ACSSU190 Year 10 Physical Sciences
Energy Conservation - Energy conservation in a system can be explained by describing energy transfers and transformations ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL115 Year 12 Maintaining the internal environment
Homeostasis - Animals, whether osmo-regulators or osmo-conformers, and plants, have various mechanisms to maintain water balance that involve structural features, and behavioural, physiological and homeostatic responses ACSCH018 Year 11 Chemical fundamentals
Properties and structure of atoms - Atoms can be modelled as a nucleus surrounded by electrons in distinct energy levels, held together by electrostatic forces of attraction between the nucleus and electrons; atoms can be represented using electron shell diagrams (all electron shells or val ACSCH025 Year 11 Chemical fundamentals
Properties and structure of materials - Materials are either pure substances with distinct measurable properties (for example, melting and boiling point, reactivity, strength, density) or mixtures with properties dependent on the identity and relative amounts of the substances that make up the ACSCH030 Year 11 Chemical fundamentals
Properties and structure of materials - Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons and protons; ions are represented by formulae which include the number of constituent atoms and the charge of the ion (for example, O2–, SO42–) ACSCH032 Year 11 Chemical fundamentals
Properties and structure of materials - The characteristic properties of metals (for example, malleability, thermal conductivity, electrical conductivity) are explained by modelling metallic bonding as a regular arrangement of positive ions (cations) made stable by electrostatic forces of attra ACSCH036 Year 11 Chemical fundamentals
Chemical reactions - All chemical reactions involve the creation of new substances and associated energy transformations, commonly observable as changes in the temperature of the surroundings and/or the emission of light ACSCH037 Year 11 Chemical fundamentals
Chemical reactions - Endothermic and exothermic reactions can be explained in terms of the Law of Conservation of Energy and the breaking and reforming of bonds; heat energy released or absorbed can be represented in thermochemical equations ACSCH039 Year 11 Chemical fundamentals
Chemical reactions - A mole is a precisely defined quantity of matter equal to Avogadro’s number of particles; the mole concept and the Law of Conservation of Mass can be used to calculate the mass of reactants and products in a chemical reaction ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH059 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - Data from chromatography techniques (for example, thin layer, gas and highperformance liquid chromatography) can be used to determine the composition and purity of substances; the separation of the components is caused by the variation of strength of the ACSCH060 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The behaviour of gases, including the qualitative relationships between pressure, temperature and volume, can be explained using kinetic theory ACSCH065 Year 11 Molecular interactions and reactions
Aqueous solutions and acidity - The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature ACSCH069 Year 11 Molecular interactions and reactions
Rates of chemical reactions - The rate of chemical reactions can be quantified by measuring the rate of formation of products or the depletion of reactants ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH131 Year 12 Structure synthesis and design
Chemical synthesis and design - Chemical synthesis involves the selection of particular reagents to form a product with specific properties (for example, pharmaceuticals, fuels, cosmetics, cleaning products) ACSPH039 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is conserved in the energy transfers and transformations that occur in an electrical circuit ACSPH042 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Power is the rate at which energy is transformed by a circuit component; power enables quantitative analysis of energy transformations in the circuit ACSPH043 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Resistance for ohmic and nonohmic components is defined as the ratio of potential difference across the component to the current in the component ACSPH060 Year 11 Linear Motion and Waves
Linear motion and force - Uniformly accelerated motion is described in terms of relationships between measurable scalar and vector quantities, including displacement, speed, velocity and acceleration ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH062 Year 11 Linear Motion and Waves
Linear motion and force - Vertical motion is analysed by assuming the acceleration due to gravity is constant near Earth’s surface ACSPH063 Year 11 Linear Motion and Waves
Linear motion and force - Newton’s Three Laws of Motion describe the relationship between the force or forces acting on an object, modelled as a point mass, and the motion of the object due to the application of the force or forces ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH066 Year 11 Linear Motion and Waves
Linear motion and force - Collisions may be elastic and inelastic; kinetic energy is conserved in elastic collisions ACSPH072 Year 11 Linear Motion and Waves
Waves - The superposition of waves in a medium may lead to the formation of standing waves and interference phenomena, including standing waves in pipes and on stretched strings ACSPH102 Year 12 Gravity and electromagnetism
Electromagnetism - Electrostatically charged objects exert a force upon one another; the magnitude of this force can be calculated using Coulomb’s Law ACSPH110 Year 12 Gravity and electromagnetism
Electromagnetism - A changing magnetic flux induces a potential difference; this process of electromagnetic induction is used in stepup and stepdown transformers, DC and AC generators, and AC induction motors ACSBL053 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Cellular respiration is a biochemical process that occurs in different locations in the cytosol and mitochondria and metabolises organic compounds, aerobically or anaerobically, to release useable energy in the form of ATP; the overall process can be repr ACSPH108 Year 12 Gravity and electromagnetism
Electromagnetism - Magnets, magnetic materials, moving charges and currentcarrying wires experience a force in a magnetic field; this force is utilised in DC electric motors ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system ACSPH100 Year 12 Gravity and electromagnetism
Gravity and motion - When an object experiences a net force of constant magnitude perpendicular to its velocity, it will undergo uniform circular motion, including circular motion on a horizontal plane and around a banked track ACSCH031 Year 11 Chemical fundamentals
Properties and structure of materials - The properties of ionic compounds (for example, high melting point, brittleness, ability to conduct electricity when liquid or in solution) are explained by modelling ionic bonding as ions arranged in a crystalline lattice structure with forces of attract ACSCH137 Year 12 Structure synthesis and design
Chemical synthesis and design - Fuels (for example, biodiesel, ethanol, hydrogen) can be synthesised from organic or inorganic sources using a range of chemical reactions including addition, oxidation and esterification ACSPH075 Year 11 Linear Motion and Waves
Waves - A ray model of light may be used to describe reflection, refraction and image formation from lenses and mirrors ACSPH077 Year 11 Linear Motion and Waves
Waves - The speed of light is finite and many orders of magnitude greater than the speed of mechanical waves (for example, sound and water waves); its intensity decreases in an inverse square relationship with distance from a point source ACSPH098 Year 12 Gravity and electromagnetism
Gravity and motion - The vector nature of the gravitational force can be used to analyse motion on inclined planes by considering the components of the gravitational force (that is, weight) parallel and perpendicular to the plane ACSPH103 Year 12 Gravity and electromagnetism
Electromagnetism - A positively charged body placed in an electric field will experience a force in the direction of the field; the strength of the electric field is defined as the force per unit charge ACSPH104 Year 12 Gravity and electromagnetism
Electromagnetism - Point charges and charged objects produce an electric field in the space that surrounds them; field theory attributes the electrostatic force on a point charge or charged body to the presence of an electric field ACSPH105 Year 12 Gravity and electromagnetism
Electromagnetism - When a charged body moves or is moved from one point to another in an electric field and its potential energy changes, work is done on or by the field
























424 low relevance results shown for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17 | Next | View 100 per page
Showing low relevance matches only. Return to normal search results
Curriculum resources related to 'for'
ACSSU005 Foundation Physical SciencesForces and Moving - The way objects move depends on a variety of factors including their size and shape ACSSU031 Year 2 Chemical Sciences
Materials - Different materials can be combined, including by mixing, for a particular purpose ACSSU033 Year 2 Physical Sciences
Forces and Moving - A push or a pull affects how an object moves or changes shape ACSSU076 Year 4 Physical Sciences
Forces and Moving - Forces can be exerted by one object on another through direct contact or from a distance ACSSU080 Year 5 Physical Sciences
Light and Sound - Light from a source forms shadows and can be absorbed, reflected and refracted ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU117 Year 7 Physical Sciences
Forces and Machines - Change to an object’s motion is caused by unbalanced forces, including Earth’s gravitational attraction, acting on the object ACSSU153 Year 8 Earth and Space Sciences
Rocks and Minerals - Sedimentary, igneous and metamorphic rocks contain minerals and are formed by processes that occur within Earth over a variety of timescales ACSSU155 Year 8 Physical Sciences
Energy Forms - Energy appears in different forms, including movement (kinetic energy), heat and potential energy, and energy transformations and transfers cause change within systems ACSSU178 Year 9 Chemical Sciences
Chemical Reactions - Chemical reactions involve rearranging atoms to form new substances; during a chemical reaction mass is not created or destroyed ACSSU225 Year 8 Chemical Sciences
Chemical Reactions - Chemical change involves substances reacting to form new substances ACSSU190 Year 10 Physical Sciences
Energy Conservation - Energy conservation in a system can be explained by describing energy transfers and transformations ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL115 Year 12 Maintaining the internal environment
Homeostasis - Animals, whether osmo-regulators or osmo-conformers, and plants, have various mechanisms to maintain water balance that involve structural features, and behavioural, physiological and homeostatic responses ACSCH018 Year 11 Chemical fundamentals
Properties and structure of atoms - Atoms can be modelled as a nucleus surrounded by electrons in distinct energy levels, held together by electrostatic forces of attraction between the nucleus and electrons; atoms can be represented using electron shell diagrams (all electron shells or val ACSCH025 Year 11 Chemical fundamentals
Properties and structure of materials - Materials are either pure substances with distinct measurable properties (for example, melting and boiling point, reactivity, strength, density) or mixtures with properties dependent on the identity and relative amounts of the substances that make up the ACSCH030 Year 11 Chemical fundamentals
Properties and structure of materials - Ions are atoms or groups of atoms that are electrically charged due to an imbalance in the number of electrons and protons; ions are represented by formulae which include the number of constituent atoms and the charge of the ion (for example, O2–, SO42–) ACSCH032 Year 11 Chemical fundamentals
Properties and structure of materials - The characteristic properties of metals (for example, malleability, thermal conductivity, electrical conductivity) are explained by modelling metallic bonding as a regular arrangement of positive ions (cations) made stable by electrostatic forces of attra ACSCH036 Year 11 Chemical fundamentals
Chemical reactions - All chemical reactions involve the creation of new substances and associated energy transformations, commonly observable as changes in the temperature of the surroundings and/or the emission of light ACSCH037 Year 11 Chemical fundamentals
Chemical reactions - Endothermic and exothermic reactions can be explained in terms of the Law of Conservation of Energy and the breaking and reforming of bonds; heat energy released or absorbed can be represented in thermochemical equations ACSCH039 Year 11 Chemical fundamentals
Chemical reactions - A mole is a precisely defined quantity of matter equal to Avogadro’s number of particles; the mole concept and the Law of Conservation of Mass can be used to calculate the mass of reactants and products in a chemical reaction ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH059 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - Data from chromatography techniques (for example, thin layer, gas and highperformance liquid chromatography) can be used to determine the composition and purity of substances; the separation of the components is caused by the variation of strength of the ACSCH060 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The behaviour of gases, including the qualitative relationships between pressure, temperature and volume, can be explained using kinetic theory ACSCH065 Year 11 Molecular interactions and reactions
Aqueous solutions and acidity - The solubility of substances in water, including ionic and molecular substances, can be explained by the intermolecular forces between species in the substances and water molecules, and is affected by changes in temperature ACSCH069 Year 11 Molecular interactions and reactions
Rates of chemical reactions - The rate of chemical reactions can be quantified by measuring the rate of formation of products or the depletion of reactants ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH131 Year 12 Structure synthesis and design
Chemical synthesis and design - Chemical synthesis involves the selection of particular reagents to form a product with specific properties (for example, pharmaceuticals, fuels, cosmetics, cleaning products) ACSPH039 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is conserved in the energy transfers and transformations that occur in an electrical circuit ACSPH042 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Power is the rate at which energy is transformed by a circuit component; power enables quantitative analysis of energy transformations in the circuit ACSPH043 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Resistance for ohmic and nonohmic components is defined as the ratio of potential difference across the component to the current in the component ACSPH060 Year 11 Linear Motion and Waves
Linear motion and force - Uniformly accelerated motion is described in terms of relationships between measurable scalar and vector quantities, including displacement, speed, velocity and acceleration ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH062 Year 11 Linear Motion and Waves
Linear motion and force - Vertical motion is analysed by assuming the acceleration due to gravity is constant near Earth’s surface ACSPH063 Year 11 Linear Motion and Waves
Linear motion and force - Newton’s Three Laws of Motion describe the relationship between the force or forces acting on an object, modelled as a point mass, and the motion of the object due to the application of the force or forces ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH066 Year 11 Linear Motion and Waves
Linear motion and force - Collisions may be elastic and inelastic; kinetic energy is conserved in elastic collisions ACSPH072 Year 11 Linear Motion and Waves
Waves - The superposition of waves in a medium may lead to the formation of standing waves and interference phenomena, including standing waves in pipes and on stretched strings ACSPH102 Year 12 Gravity and electromagnetism
Electromagnetism - Electrostatically charged objects exert a force upon one another; the magnitude of this force can be calculated using Coulomb’s Law ACSPH110 Year 12 Gravity and electromagnetism
Electromagnetism - A changing magnetic flux induces a potential difference; this process of electromagnetic induction is used in stepup and stepdown transformers, DC and AC generators, and AC induction motors ACSBL053 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Cellular respiration is a biochemical process that occurs in different locations in the cytosol and mitochondria and metabolises organic compounds, aerobically or anaerobically, to release useable energy in the form of ATP; the overall process can be repr ACSPH108 Year 12 Gravity and electromagnetism
Electromagnetism - Magnets, magnetic materials, moving charges and currentcarrying wires experience a force in a magnetic field; this force is utilised in DC electric motors ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system ACSPH100 Year 12 Gravity and electromagnetism
Gravity and motion - When an object experiences a net force of constant magnitude perpendicular to its velocity, it will undergo uniform circular motion, including circular motion on a horizontal plane and around a banked track ACSCH031 Year 11 Chemical fundamentals
Properties and structure of materials - The properties of ionic compounds (for example, high melting point, brittleness, ability to conduct electricity when liquid or in solution) are explained by modelling ionic bonding as ions arranged in a crystalline lattice structure with forces of attract ACSCH137 Year 12 Structure synthesis and design
Chemical synthesis and design - Fuels (for example, biodiesel, ethanol, hydrogen) can be synthesised from organic or inorganic sources using a range of chemical reactions including addition, oxidation and esterification ACSPH075 Year 11 Linear Motion and Waves
Waves - A ray model of light may be used to describe reflection, refraction and image formation from lenses and mirrors ACSPH077 Year 11 Linear Motion and Waves
Waves - The speed of light is finite and many orders of magnitude greater than the speed of mechanical waves (for example, sound and water waves); its intensity decreases in an inverse square relationship with distance from a point source ACSPH098 Year 12 Gravity and electromagnetism
Gravity and motion - The vector nature of the gravitational force can be used to analyse motion on inclined planes by considering the components of the gravitational force (that is, weight) parallel and perpendicular to the plane ACSPH103 Year 12 Gravity and electromagnetism
Electromagnetism - A positively charged body placed in an electric field will experience a force in the direction of the field; the strength of the electric field is defined as the force per unit charge ACSPH104 Year 12 Gravity and electromagnetism
Electromagnetism - Point charges and charged objects produce an electric field in the space that surrounds them; field theory attributes the electrostatic force on a point charge or charged body to the presence of an electric field ACSPH105 Year 12 Gravity and electromagnetism
Electromagnetism - When a charged body moves or is moved from one point to another in an electric field and its potential energy changes, work is done on or by the field
Products related to 'for'

Vernier Bar Tape Guide
VERNIER BAR TAPE GUIDE
The Bar Tape Guide contains an adaptor that allows Bar Tape to be used with a Vernier Photogate. Bar Tape is 3 m long, and it has alternating opaque and transparent bars that have a spacing of 1.524 cm. The tape can be attached to objects and pulled...
Order code: TAPE-GUIDE

Vernier Tris-Compatible Flat pH Electrode Only
VERNIER TRIS-COMPATIBLE FLAT PH ELECTRODE ONLY
This is an electrode only and connects via BNC to one of Vernier's Electrode Amplifiers to function as a pH sensor.
It is not compatible with the Vernier PH-BTA pH Sensor
Combined with the EA-BTA Electrode Amplifier it b...
Order code: FPH-BNC

Vernier Spirometer Disposable Bacterial Filter - Pack of 10
VERNIER DISPOSABLE BACTERIAL FILTER
Use of the single use, disposable microbacterial filter significantly reduces how often you will need to sterilise your spirometer's flow head. The filter's 30 mm ID opening allows for an easy, air-tight fit to the flow head.
Each fi...
Order code: SPR-FIL10

Vernier Spirometer Disposable Mouth Piece - Pack of 30
VERNIER DISPOSABLE MOUTH PIECE
For a more sterile environment, use these single use, cardboard mouthpieces to help eliminate the spreading of contagious material between students.
The mouth piece is for use by a single person only.
Tube dimensions:
~31.5mmID
~33mm...
Order code: SPR-MP30

Vernier Spirometer Flow Head
VERNIER SPIROMETER FLOW HEAD
An extra flow head eliminates the need for equipment downtime while sterilising your original flow head. The flow head consists of a polycarbonate outer body with a stainless-steel inner mesh.
Order code: SPR-FLOW

Vernier Turbidity Cuvette Standard
VERNIER STANDARD TURBIDITY CUVETTE
Replacement kit of 100 NTU turbidity standard and one empty glass cuvette for the TRB-BTA Vernier Turbidity Sensor.
Order code: TRB-ACC

Agricultural Science with Vernier - Electronic Version
AGRICULTURAL SCIENCE WITH VERNIER - ELECTRONIC
Agricultural Science with Vernier is a digital lab book containing a collection of 29 experiments that can be useful in teaching agricultural science at the high school or college level. Some experiments are designed to be do...
Order code: AWV-E

Vernier Investigating Chemistry through Inquiry
VERNIER INVESTIGATING CHEMISTRY THROUGH INQUIRY
Investigating Chemistry through Inquiry lab book contains 25 inquiry-based chemistry investigations.
Each experiment includes a preliminary activity, teacher information, sample researchable questions and sample data for ...
Order code: CHEM-I

Vernier Investigating Chemistry through Inquiry - Electronic Version
VERNIER INVESTIGATING CHEMISTRY THROUGH INQUIRY - ELECTRONIC
Investigating Chemistry through Inquiry lab book contains 25 inquiry-based chemistry investigations.
Each experiment includes a preliminary activity, teacher information, sample researchable questions and sam...
Order code: CHEM-I-E

Vernier Physics Explorations and Projects
VERNIER PHYSICS EXPLORATIONS AND PROJECTS
Vernier Physics Explorations and Projects is a collection of laboratory investigations aligned to the USA Next Generation Science Standards (NGSS). Most included investigations set up a situation for students to explore and analyz...
Order code: PEP

Vernier Physics Explorations and Projects - Electronic Version
VERNIER PHYSICS EXPLORATIONS AND PROJECTS - ELECTRONIC
Vernier Physics Explorations and Projects is a collection of laboratory investigations aligned to the USA Next Generation Science Standards (NGSS). Most included investigations set up a situation for students to explo...
Order code: PEP-E

Vernier Hands-On Introduction to NI LabVIEW
Free e-Book Download
VERNIER HANDS-ON INTRODUCTION TO NI LABVIEW
A discontinued book that is still available as a free download here: http://www2.vernier.com/free/hands-on-introduction-to-ni-labview-with-vernier.zip
An introduction to NI LabVIEW programming through a series of hands-on ex...
Order code: LWV

STEM with Vernier and Lego Mindstorm
Discontinued. Only 1 still available.
STEM WITH VERNIER AND LEGO MINDSTORM
Hands-on learning is the best way to attract students into Science, Technology, Engineering, and Math (STEM) careers. When used with the LEGO NXT Adaptor, this new book makes it easy for science and engineering teachers to integrate th...
Order code: STEM

Biological Microscope with LED Lighting
BIOLOGICAL (Monocular) MICROSCOPE WITH RECHARGEABLE BATTERIES
This high quality monocular microscope with LED lighting is ideal for both field and lab use. This microscope can be powered by rechargeable batteries, mains power or a combination of both. It is suitable for ...
Order code: MMLED
| Purchase QTY: (Each) | 1+ |
|---|---|
| Scientrific's price | $316.00 |
| Educational price | $306.00 |
| CLICK FOR QTY PRICING | |
| Prices exclude GST and freight | |

Stereo Microscope 20X and 40X with LED Light
STEREO MICROSCOPE WITH RECHARGEABLE BATTERIES
A high quality stereo microscope with LED lighting that is ideal for field use as it can be powered by the included rechargeable batteries. In the lab it can be powered by the rechargeable batteries, by mains power or a comb...
Order code: MS24LED
| Purchase QTY: (Each) | 1+ |
|---|---|
| Scientrific's price | $365.00 |
| Educational price | $355.00 |
| CLICK FOR QTY PRICING | |
| Prices exclude GST and freight | |

Vernier Green Diffraction Laser
VERNIER GREEN DIFFRACTION LASER
This Class 2 Green Diffraction Laser is an optional accessory for the Diffraction Apparatus. Operating at 532nm, it allows students to see and measure how diffraction patterns depend on light wavelength.
Note: the Vernier Green Diffract...
Order code: GDL-DAK

Vernier LabQuest Mini Physics Starter Package
VERNIER LABQUEST MINI PHYSICS STARTER PACKAGE
Designed for use by a group of 2-4 students, the Vernier LabQuest Mini Physics Starter Package includes:
• LQ-MINI Vernier LabQuest Mini Interface
•
Order code: LM-PHY-ST

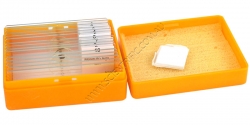
Microscope Slide Set of 10 Anatomy Slides plus Blanks
A set of ten prepared and numbered whole mount (w.m.) microscope slides plus 5 blank microscope slides and 5 cover slips. Supplied in a sturdy plastic case with a squeeze-to-release lid and packed in a cardboard box for shipping.
The prepared slides are:
1. Feathers
2...
Order code: SC92038

Vernier LabQuest Mini Physics Deluxe Package
VERNIER LABQUEST MINI PHYSICS DELUXE PACKAGE
Designed for use by a group of 2-4 students, the Vernier LabQuest Mini Physics Deluxe Package includes:
• LQ-MINI Vernier LabQuest Mini Interface
•
Order code: LM-PHY-DX

Microscope Slide Set of 20 Mixed Slides plus 40 Blanks
A set of 20 prepared and numbered mixed biology microscope slides supplied in a sturdy plastic case with a squeeze-to-release lid.
Also included are 40 blank slides and cover slips in a separate cardboard box.
The prepared slides are:
1. Drosophila (larval form) w.m...
Order code: SC92040

Microscope Slide Set of 25 Botany Slides
A set of 25 prepared and numbered microscope slides for use in botany and zoology. Supplied in a sturdy plastic case with a squeeze-to-release lid and packed in a cardboard box for shipping.
The prepared slides are:
1. Onion root tip mitosis l.s.
2. Onion chromosome w...
Order code: SC92041

Stirling Engine Model
Power a Car with Solar Generated Electricity. Discover the Stirling engine, a simple, clean and efficient energy technology that is quickly becoming a viable source of electricity as the availability of fossil fuels declines. The Stirling engine in this kit uses renewable energy ...
Order code: 620325

Bubble Science (V2.0)
SCIENCE EXPERIMENTS WITH SOAP BUBBLES.
Experiment with fascinating soap bubbles to learn physical science fundamentals in a fun way.
For countless generations people have been captivated by the unique properties of soap bubbles. See for yourself why these elegantly s...
Order code: 665043

Microcontroller Computer Systems Engineering Kit
Last kit available
COMPUTER SYSTEMS ENGINEERING KIT.
Microcontrollers are small, self-contained computers which are the "brains" inside dozens of the devices and appliances in your home. Microwaves, washing machines, telephones and stereos all have microcontrollers inside them. A microcon...
Order code: 614515

Milestones in Science
The 100 Most Significant Experiments and Discoveries of All Time.
Embark on an active research expedition through the history of science and technology with this Thames and Kosmos kit. Learn about famous scientists and inventors, like Archimedes, Copernicus, Einstein, W...
Order code: 625719
424 low relevance results shown for 'for'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17 | Next | View 100 per page



 ,
,