541 low relevance results shown for 'Red'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17|18|19|20|21|22 | Next | View 100 per page
Showing low relevance matches only. Return to normal search results
DNA - The transmission of heritable characteristics from one generation to the next involves DNA and genes ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU115 Year 7 Earth and Space Sciences
Earth Moon Sun - Predictable phenomena on Earth, including seasons and eclipses, are caused by the relative positions of the sun, Earth and the moon ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL085 Year 12 Heredity and continuity of life
DNA genes and the continuity of life - Frequencies of genotypes and phenotypes of offspring can be predicted using probability models, including Punnett squares, and by taking into consideration patterns of inheritance, including the effects of dominant, autosomal and sex-linked alleles and mu ACSBL090 Year 12 Heredity and continuity of life
Continuity of life on Earth - Natural selection occurs when selection pressures in the environment confer a selective advantage on a specific phenotype to enhance its survival and reproduction; this results in changes in allele frequency in the gene pool of a population ACSBL091 Year 12 Heredity and continuity of life
Continuity of life on Earth - In additional to environmental selection pressures, mutation, gene flow and genetic drift can contribute to changes in allele frequency in a population gene pool and results in microevolutionary change ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH073 Year 11 Molecular interactions and reactions
Rates of chemical reactions - Catalysts, including enzymes and metal nanoparticles, affect the rate of certain reactions by providing an alternative reaction pathway with a reduced activation energy, hence increasing the proportion of collisions that lead to a chemical change ACSCH091 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Over time, physical changes and reversible chemical reactions reach a state of dynamic equilibrium in a closed system, with the relative concentrations of products and reactants defining the position of equilibrium ACSCH096 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Equilibrium position can be predicted qualitatively using equilibrium constants ACSCH097 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acids are substances that can act as proton (hydrogen ion) donors and can be classified as monoprotic or polyprotic depending on the number of protons donated by each molecule of the acid ACSCH098 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The strength of acids is explained by the degree of ionisation at equilibrium in aqueous solution, which can be represented with chemical equations and equilibrium constants (Ka) ACSCH099 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The relationship between acids and bases in equilibrium systems can be explained using the Brønsted Lowry model and represented using chemical equations that illustrate the transfer of hydrogen ions ACSCH100 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The pH scale is a logarithmic scale and the pH of a solution can be calculated from the concentration of hydrogen ions; Kw can be used to calculate the concentration of hydrogen ions from the concentration of hydroxide ions in a solution ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH102 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Volumetric analysis methods involving acidbase reactions rely on the identification of an equivalence point by measuring the associated change in pH, using chemical indicators or pH meters, to reveal an observable end point ACSCH103 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - A range of reactions, including displacement reactions of metals, combustion, corrosion, and electrochemical processes, can be modelled as redox reactions involving oxidation of one substance and reduction of another substance ACSCH104 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Oxidation can be modelled as the loss of electrons from a chemical species, and reduction can be modelled as the gain of electrons by a chemical species; these processes can be represented using half equations ACSCH106 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - The relative strength of oxidising and reducing agents can be determined by comparing standard electrode potentials ACSCH107 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Electrochemical cells, including galvanic and electrolytic cells, consist of oxidation and reduction half reactions connected via an external circuit that allows electrons to move from the anode (oxidation reaction) to the cathode (reduction reaction) ACSCH108 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Galvanic cells, including fuel cells, generate an electrical potential difference from a spontaneous redox reaction; they can be represented as cell diagrams including anode and cathode halfequations ACSCH110 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Cell potentials at standard conditions can be calculated from standard electrode potentials; these values can be used to compare cells constructed from different materials ACSCH130 Year 12 Structure synthesis and design
Properties and structure of organic materials - Data from analytical techniques, including mass spectrometry, xray crystallography and infrared spectroscopy, can be used to determine the structure of organic molecules, often using evidence from more than one technique ACSPH040 Year 11 Thermal nuclear and electrical physics
Electrical circuits - The energy available to charges moving in an electrical circuit is measured using electric potential difference, which is defined as the change in potential energy per unit charge between two defined points in the circuit ACSPH041 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is required to separate positive and negative charge carriers; charge separation produces an electrical potential difference that can be used to drive current in circuits ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH073 Year 11 Linear Motion and Waves
Waves - A mechanical system resonates when it is driven at one of its natural frequencies of oscillation; energy is transferred efficiently into systems under these conditions ACSPH076 Year 11 Linear Motion and Waves
Waves - A wave model explains a wide range of lightrelated phenomena including reflection, refraction, total internal reflection, dispersion, diffraction and interference; a transverse wave model is required to explain polarisation ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system





















541 low relevance results shown for 'Red'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17|18|19|20|21|22 | Next | View 100 per page
Showing low relevance matches only. Return to normal search results
Curriculum resources related to 'Red'
ACSSU184 Year 10 Biological SciencesDNA - The transmission of heritable characteristics from one generation to the next involves DNA and genes ACSSU097 Year 6 Physical Sciences
Electrical Circuits - Electrical energy can be transferred and transformed in electrical circuits and can be generated from a range of sources ACSSU115 Year 7 Earth and Space Sciences
Earth Moon Sun - Predictable phenomena on Earth, including seasons and eclipses, are caused by the relative positions of the sun, Earth and the moon ACSSU229 Year 10 Physical Sciences
Forces and Motion - The motion of objects can be described and predicted using the laws of physics ACSBL029 Year 11 Biodiversity and the interconnectedness of life
Ecosystem dynamics - Models of ecosystem interactions (for example, food webs, successional models) can be used to predict the impact of change and are based on interpretation of and extrapolation from sample data (for example, data derived from ecosystem surveying techniques ACSBL085 Year 12 Heredity and continuity of life
DNA genes and the continuity of life - Frequencies of genotypes and phenotypes of offspring can be predicted using probability models, including Punnett squares, and by taking into consideration patterns of inheritance, including the effects of dominant, autosomal and sex-linked alleles and mu ACSBL090 Year 12 Heredity and continuity of life
Continuity of life on Earth - Natural selection occurs when selection pressures in the environment confer a selective advantage on a specific phenotype to enhance its survival and reproduction; this results in changes in allele frequency in the gene pool of a population ACSBL091 Year 12 Heredity and continuity of life
Continuity of life on Earth - In additional to environmental selection pressures, mutation, gene flow and genetic drift can contribute to changes in allele frequency in a population gene pool and results in microevolutionary change ACSCH056 Year 11 Molecular interactions and reactions
Intermolecular forces and gases - The shapes of molecules can be explained and predicted using three dimensional representations of electrons as charge clouds and using valence shell electron pair repulsion (VSEPR) theory ACSCH073 Year 11 Molecular interactions and reactions
Rates of chemical reactions - Catalysts, including enzymes and metal nanoparticles, affect the rate of certain reactions by providing an alternative reaction pathway with a reduced activation energy, hence increasing the proportion of collisions that lead to a chemical change ACSCH091 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Over time, physical changes and reversible chemical reactions reach a state of dynamic equilibrium in a closed system, with the relative concentrations of products and reactants defining the position of equilibrium ACSCH096 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Equilibrium position can be predicted qualitatively using equilibrium constants ACSCH097 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acids are substances that can act as proton (hydrogen ion) donors and can be classified as monoprotic or polyprotic depending on the number of protons donated by each molecule of the acid ACSCH098 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The strength of acids is explained by the degree of ionisation at equilibrium in aqueous solution, which can be represented with chemical equations and equilibrium constants (Ka) ACSCH099 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The relationship between acids and bases in equilibrium systems can be explained using the Brønsted Lowry model and represented using chemical equations that illustrate the transfer of hydrogen ions ACSCH100 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - The pH scale is a logarithmic scale and the pH of a solution can be calculated from the concentration of hydrogen ions; Kw can be used to calculate the concentration of hydrogen ions from the concentration of hydroxide ions in a solution ACSCH101 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Acidbase indicators are weak acids or bases where the acidic form is of a different colour to the basic form ACSCH102 Year 12 Equilibrium acids and redox reactions
Chemical equilibrium systems - Volumetric analysis methods involving acidbase reactions rely on the identification of an equivalence point by measuring the associated change in pH, using chemical indicators or pH meters, to reveal an observable end point ACSCH103 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - A range of reactions, including displacement reactions of metals, combustion, corrosion, and electrochemical processes, can be modelled as redox reactions involving oxidation of one substance and reduction of another substance ACSCH104 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Oxidation can be modelled as the loss of electrons from a chemical species, and reduction can be modelled as the gain of electrons by a chemical species; these processes can be represented using half equations ACSCH106 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - The relative strength of oxidising and reducing agents can be determined by comparing standard electrode potentials ACSCH107 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Electrochemical cells, including galvanic and electrolytic cells, consist of oxidation and reduction half reactions connected via an external circuit that allows electrons to move from the anode (oxidation reaction) to the cathode (reduction reaction) ACSCH108 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Galvanic cells, including fuel cells, generate an electrical potential difference from a spontaneous redox reaction; they can be represented as cell diagrams including anode and cathode halfequations ACSCH110 Year 12 Equilibrium acids and redox reactions
Oxidation and reduction - Cell potentials at standard conditions can be calculated from standard electrode potentials; these values can be used to compare cells constructed from different materials ACSCH130 Year 12 Structure synthesis and design
Properties and structure of organic materials - Data from analytical techniques, including mass spectrometry, xray crystallography and infrared spectroscopy, can be used to determine the structure of organic molecules, often using evidence from more than one technique ACSPH040 Year 11 Thermal nuclear and electrical physics
Electrical circuits - The energy available to charges moving in an electrical circuit is measured using electric potential difference, which is defined as the change in potential energy per unit charge between two defined points in the circuit ACSPH041 Year 11 Thermal nuclear and electrical physics
Electrical circuits - Energy is required to separate positive and negative charge carriers; charge separation produces an electrical potential difference that can be used to drive current in circuits ACSPH061 Year 11 Linear Motion and Waves
Linear motion and force - Representations, including graphs and vectors, and/or equations of motion, can be used qualitatively and quantitatively to describe and predict linear motion ACSPH064 Year 11 Linear Motion and Waves
Linear motion and force - Momentum is a property of moving objects; it is conserved in a closed system and may be transferred from one object to another when a force acts over a time interval ACSPH065 Year 11 Linear Motion and Waves
Linear motion and force - Energy is conserved in isolated systems and is transferred from one object to another when a force is applied over a distance; this causes work to be done and changes to kinetic and/or potential energy of objects ACSPH073 Year 11 Linear Motion and Waves
Waves - A mechanical system resonates when it is driven at one of its natural frequencies of oscillation; energy is transferred efficiently into systems under these conditions ACSPH076 Year 11 Linear Motion and Waves
Waves - A wave model explains a wide range of lightrelated phenomena including reflection, refraction, total internal reflection, dispersion, diffraction and interference; a transverse wave model is required to explain polarisation ACSPH021 Year 11 Thermal nuclear and electrical physics
Heating processes - Change of state involves internal energy changes to form or break bonds between atoms or molecules; latent heat is the energy required to be added to or removed from a system to change the state of the system
Products related to 'Red'

IEC Geiger Counter Rate Internal GM Tube
IEC GEIGER COUNTER RATEMETER WITH INTERNAL GEIGER MULLER TUBE
A fully portable Geiger Counter with an inbuilt Geiger Muller tube and LCD display powered by 3xAA batteries.
The instrument is light, very robust and is useful for both classroom and field work.
The larg...
Order code: AP1884-001

IEC Geiger Counter Ratemeter No GM Tube
IEC GEIGER COUNTER AND RATE METER WITHOUT GM TUBE
A fully portable Geiger Counter with LCD display powered by 3xAA batteries. The instrument is light, very robust and is useful for both classroom and field work.
It does NOT include a Geiger Muller Tube which can be orde...
Order code: AP1884-002

IEC Mass of Electron Apparatus
IEC MASS OF ELECTRON APPARATUS
The IEC Mass of Electron apparatus is a 'Magic Eye' tube mounted on a base with terminals to accept the heater voltage. Safety leads terminated in safety banana plugs can be connected to a high voltage source for the plate voltage.
When ...
Order code: AP2120-001

IEC Millikans Apparatus No Power Supply
IEC MILLIKAN'S APPARATUS WITHOUT POWER SUPPLY
This apparatus is used to perform Millikan's experiment with charged particles moving in an electric field or to observe Brownian motion. Complete with a telescope and graticule for focusing through one of the two windows prov...
Order code: AP2130-001

IEC Millikans Apparatus with Power Supply
IEC MILLIKAN'S APPARATUS WITH POWER SUPPLY
This apparatus is used to perform Millikan's experiment with charged particles moving in an electric field or to observe Brownian motion. Complete with a telescope and graticule for focusing through one of the two windows provide...
Order code: AP2131-001

IEC Photo-Electric Effect LED Planck's Constant Apparatus
IEC PHOTOELECTRIC EFFECT LED PLANCK'S CONSTANT
A version of IEC's Photo Electric Effect with LED light sources and two digital meters to simultaneously display both the current through the internal photo-cell in nanoamps and the backing voltage applied to the cell to brin...
Order code: AP2342-001

IEC Planck's Constant - Low Cost Apparatus
IEC PLANCK'S CONSTANT SIMPLE APPARATUS
A low cost instrument used to determine Planck’s Constant and discover that the wavelength of light determines the energy in the photons … not the amount of light.
The voltage that causes 2 microamps to flow through each selected ...
Order code: AP2343-001

IEC Wilson's Cloud Chamber
IEC WILSON'S CLOUD CHAMBER
Alcohol saturates a pad in the IEC Wilson's Cloud Chamber and a vacuum pump (reversed tyre pump) is used to draw a partial vacuum to super saturate the remaining air with alcohol vapour.
An Alpha 'pin source' (not included) placed in the cham...
Order code: AP4660-001

Kit for use with Circuit Putty
Circuit Putty can be used to create tools and activities that allow kids of all ages to experiment with circuits and electronics using conductive dough.
Description
Using the provided unique recipe, you can create a batch of non-toxic, conductive dough! It's super sim...
Order code: BDGCP

ProScope C Mount Adapter with IR Cut Filter
PROSCOPE C-MOUNT ADAPTER WITH IR CUT FILTER
A quality infrared cut filter. When used with your ProScope it adapts the proprietary ProScope mounting thread to an industry standard, female, 25.4mm C-Mount thread.
With the IR filter fitted a ProScope can be easily fitted...
Order code: BT-CMT-IRC

Slim Edge Light Pad
A Light Pad for illuminating microscope slides, it is especially useful when viewing slides or other transparent objects with handheld or digital microscope cameras.
Powered by 6 x AAA batteries (not included) or the included mains power supply.
Includes soft cover....
Order code: BT-PAD

Vernier Chemical Polarimeter Accessory Cells
VERNIER CHEMICAL POLARIMETER CELLS
A set of four replacement sample cells for both the CHEM-POL Vernier Chemical Polarimeter and the GDX-POL Vernier Go Direct Polarimeter. These are the same sample cells as the one that ships with Vernier polarimeters. They are designed t...
Order code: CELLS-POL
IEC Clamp Safety for Gas Syringe 100ml
IEC SAFETY CLAMP FOR GAS SYRINGES
A simple safety device for holding a gas syringe, supporting the body and the
plunger to prevent the plunger from extending beyond a certain point. Gas syringes are fragile and expensive and this clamp reduces breakages, reduces experime...
Order code: CH0860-001

IEC Colorimeter Digital Kit 9V
IEC 9V DIGITAL COLORIMETER KIT
The IEC Colorimeter is a high quality student meter for measuring concentrations of chemical solutions. The large LCD screen can display either 'Transmission' or 'Absorption' measurements.
Zero and calculations are performed automatically...
Order code: CH1003-001
IEC Filter Pump Hose Fitting Brass Non-Retentive Valve
IEC FILTER PUMP HOSE FITTING BRASS NON-RETURN VALVE
A compact filter pump of nickel plated brass construction for attaching to a mains water supply to provide a vacuum to -80kPa for assisting filtering in the laboratory.
The side outlet connects to the flask containing...
Order code: CH1852-001
IEC Filter Pump Tap Fitting Brass Non-Retentive Valve
IEC FILTER PUMP TAP FITTING BRASS NON-RETURN VALVE
A compact filter pump of nickel plated brass construction for attaching to a mains water supply to provide a vacuum to -80kPa for assisting filtering in the laboratory.
The side outlet connects to the flask containing ...
Order code: CH1853-001

Vernier Connected Science System
Product is purchased by selecting the components that you require.
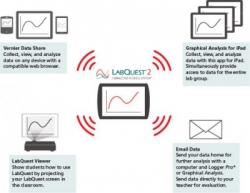
Collect Once, Analyze Anywhere The Connected Science System is not a single product, it is a networked collection of technology that supports hands-on, collaborative learning with individualised accountability. It comprises the following products: LabQuest 2 Order code: CSS
Collect Once, Analyze Anywhere The Connected Science System is not a single product, it is a networked collection of technology that supports hands-on, collaborative learning with individualised accountability. It comprises the following products: LabQuest 2 Order code: CSS

Vernier Precision Volume Dispenser
VERNIER PRECISION VOLUME DISPENSER
The Precision Volume Dispenser offers highly accurate and controlled drop dispensing for automated titrations. When paired with a drop counter, it improves efficiency and precision, delivering consistent results with reduced errors compa...
Order code: DC-DISP

Vernier DCU Terminal Plug Kit
VERNIER DCU TERMINAL PLUG KIT
The screw terminal plug connector is a detachable wiring plug for your Vernier Digital Control Unit (DCU). The DCU is shipped with one plug but it may be useful to have extras.
Store your favourite projects with the electronics wired to t...
Order code: DCU-PLUG

Vernier Dynamics Cart and Track System with Motion Encoder
VERNIER DYNAMICS CART AND TRACK SYSTEM WITH MOTION ENCODER
The Dynamics Cart and Track System with Motion Encoder is a revolutionary way for physics students to study dynamics. The Motion Encoder adds an optical position sensing system to record cart motion and eliminates...
Order code: DTS-EC

Vernier Dynamics Track System Encoder Long
VERNIER DYNAMICS TRACK SYSTEM WITH MOTION ENCODER LONG
The Dynamics Cart and Track System with Motion Encoder is a revolutionary way for physics students to study dynamics. The Motion Encoder adds an optical position sensing system to record cart motion and eliminates the...
Order code: DTS-EC-LONG

Vernier Electrochemistry Metals Kit
VERNIER ELECTROCHEMISTRY METALS KIT.
The Electrochemistry Metals Kit provides a convenient, reusable set of seven different metal samples for conducting electrochemical experiments. When paired with a voltage probe, this kit allows students to easily study oxidation, redu...
Order code: ECHEM-MTLS

Vernier Electrochemistry Half-Cell Plate
VERNIER ELECTROCHEMISTRY HALF-CELL PLATE.
The Electrochemistry Half-Cell Plate is a reusable, pre-measured platform designed to simplify electrochemical experiments with multiple metal-ion half cells. Its innovative design allows for precise measurements and easy setup, m...
Order code: ECHEM-PLT

IEC AC Circuits Experiment Set
IEC AC CIRCUITS EXPERIMENT SET
The IEC AC Circuits Experiment Set is a convenient, high power set of inductors, capacitors and resistors
for voltages up to 240V AC mounted in a sturdy well-ventilated housing with carry handles.
This passive (unpowered) instrument can ...
Order code: EM0050-001

IEC Electricity Kit AC Theory Unit
IEC ELECTRICITY KIT AC THEORY UNIT
The IEC AC Theory Electricity Kit contains all the components necessary to perform a range of experiments for teaching AC electrical theory. The kit contains an accurate digital signal source so students can study the effects of frequenc...
Order code: EM0060-001
541 low relevance results shown for 'Red'. Prev |1|2|3|4|5|6|7|8|9|10|11|12|13|14|15|16|17|18|19|20|21|22 | Next | View 100 per page



 ,
,